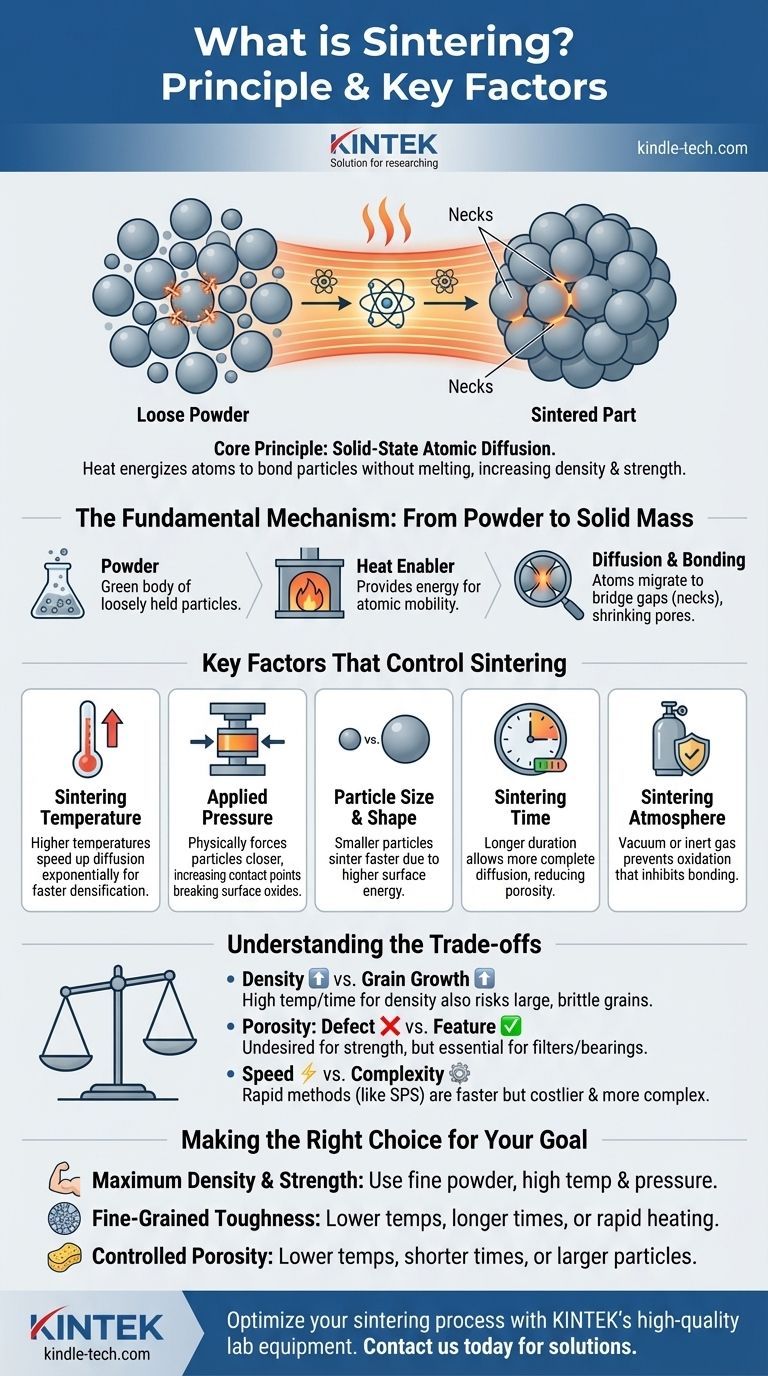
At its core, sintering is a thermal treatment process that transforms a collection of individual particles into a solid, dense object. It accomplishes this by applying heat and often pressure, but crucially, at temperatures below the material's melting point. Instead of melting and fusing, the atoms themselves migrate across the boundaries of the particles, effectively knitting them together into a single, coherent mass.
The central principle of sintering is not melting, but solid-state atomic diffusion. By energizing atoms with heat, the process reduces the empty space between particles, bonding them directly to increase the material's density and strength.

The Fundamental Mechanism: Atomic Diffusion
Sintering is a fascinating process that occurs on a microscopic level. Understanding how loose powder becomes a solid part is key to controlling the outcome.
From Powder to a Coherent Mass
The starting point is a mass of individual particles, often compacted into a desired shape called a "green body." At this stage, the particles are only held together by weak mechanical forces, and the object has very low strength.
The Role of Heat as an Enabler
Heat provides the critical energy for sintering. It doesn't melt the material, but it makes the atoms within the particle structure vibrate and become mobile enough to move. This mobility is the key to the entire process.
How Atoms Bridge the Gaps
Where two particles touch, a boundary exists. With enough thermal energy, atoms begin to migrate or diffuse across this boundary. This movement slowly forms a solid bridge, or "neck," between the particles. As time goes on, these necks grow, pulling the particle centers closer together and gradually shrinking the empty pores between them.
Key Factors That Control the Sintering Process
To achieve a desired outcome, materials engineers must precisely manipulate several variables. Each factor has a direct impact on the final properties of the sintered part.
Sintering Temperature
This is the most dominant factor. Higher temperatures increase the rate of atomic diffusion exponentially, leading to faster and more complete densification. However, the temperature must remain below the material's melting point.
Applied Pressure
Applying external pressure physically forces the particles closer together. This increases the number of contact points where diffusion can occur and can help break down any surface oxide layers that might inhibit bonding.
Particle Size and Shape
Smaller particles sinter faster and at lower temperatures. This is because a greater proportion of their atoms are on the surface, creating a higher driving force for the system to reduce its total surface energy by bonding together.
Sintering Time
Sintering is not instantaneous. Diffusion requires time. A longer duration at the sintering temperature allows the diffusion process to continue, further reducing porosity and increasing the density and strength of the final object.
Sintering Atmosphere
The gas surrounding the material during sintering is critical. A vacuum or an inert gas (like argon) is often used to prevent oxidation, which can interfere with the bonding process. In some cases, a reactive atmosphere is used to achieve specific chemical changes.
Understanding the Trade-offs
Optimizing the sintering process always involves balancing competing factors. There is no single "best" setting; the ideal parameters depend entirely on the desired properties of the final product.
Density vs. Grain Growth
While high temperatures and long times are excellent for achieving maximum density, they also encourage grain growth. This is a phenomenon where smaller crystal grains within the material merge into larger ones. Excessively large grains can often make a material more brittle, so there is a trade-off between achieving full density and maintaining a fine, strong microstructure.
Porosity: Defect or Desired Feature?
In many applications, such as structural steel parts, porosity is a defect to be eliminated. However, in other cases, it is the desired outcome. Sintering is used to create porous metals for filters or self-lubricating bearings, where the controlled empty space is essential to the part's function.
Speed vs. Complexity
Conventional sintering in a furnace is a relatively slow process. Advanced techniques like Spark Plasma Sintering (SPS) can densify materials in a matter of minutes. The trade-off is a significant increase in equipment cost and complexity.
Making the Right Choice for Your Goal
The ideal sintering strategy is dictated by the intended application of the final component. Your approach should be tailored to achieve the specific properties you need.
- If your primary focus is maximum density and strength: Use fine starting powders and a combination of high temperature and sufficient pressure to eliminate as much porosity as possible.
- If your primary focus is preserving a fine-grained microstructure for toughness: Consider lower sintering temperatures for longer times or investigate advanced, rapid heating techniques that limit the time available for grain growth.
- If your primary focus is creating a controlled porous structure: Deliberately use lower temperatures, shorter times, or larger starting particles to achieve partial bonding without fully closing the gaps between them.
Ultimately, mastering sintering is about understanding and controlling atomic movement to build stronger materials from the particle up.
Summary Table:
| Factor | Key Influence on Sintering |
|---|---|
| Sintering Temperature | Drives atomic diffusion rate; higher temperatures increase densification speed. |
| Applied Pressure | Forces particles closer, increasing contact points and aiding bonding. |
| Particle Size & Shape | Smaller particles sinter faster and at lower temperatures due to higher surface energy. |
| Sintering Time | Longer durations allow for more complete diffusion, increasing density and strength. |
| Sintering Atmosphere | Prevents oxidation (e.g., vacuum, inert gas) or enables specific chemical reactions. |
Ready to optimize your sintering process for superior material performance? KINTEK specializes in high-quality lab equipment and consumables, including sintering furnaces and powder handling tools. Our experts can help you select the right equipment to achieve your specific goals, whether it's maximum density, controlled porosity, or a fine-grained microstructure. Contact our team today to discuss your laboratory's sintering needs and discover how KINTEK's solutions can enhance your research and production outcomes.
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the advantages of using a vacuum hot pressing sintering furnace? Superior Density for Nanocrystalline Fe3Al
- What critical processing conditions does a vacuum hot pressing sintering furnace provide for high-density VC/Cu?
- What are the advantages of using a vacuum hot pressing furnace? Achieve 98.9% Density in Al2O3-TiC Laminated Ceramics
- What is the primary function of a vacuum hot pressing sintering furnace? Expert Guide for Ti-22Al-25Nb Fabrication
- What are the advantages of vacuum sintering? Achieve Superior Purity, Strength, and Performance



















