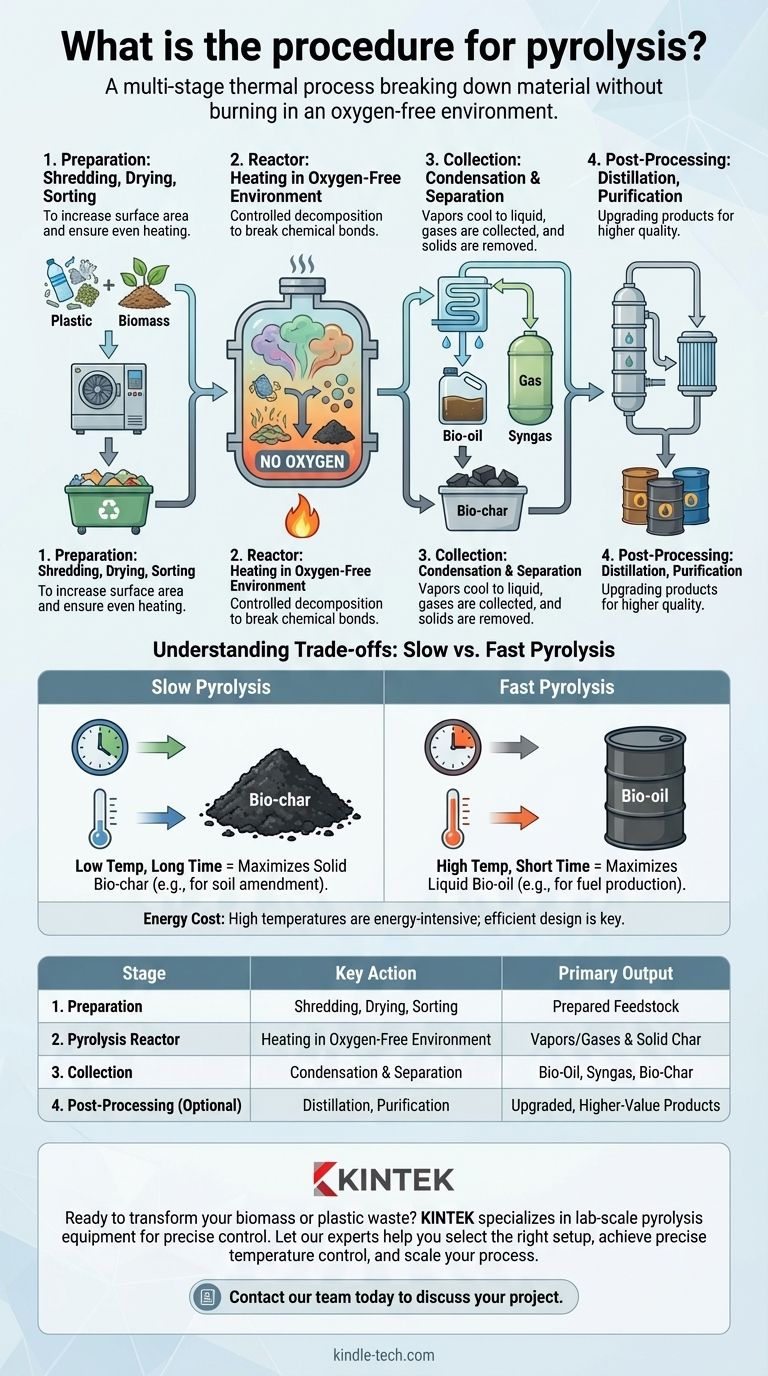
In essence, the procedure for pyrolysis is a multi-stage thermal process that breaks down material like plastic or biomass without burning it. It begins with preparing the material (shredding and drying), followed by heating it in a sealed, oxygen-free reactor to trigger decomposition. Finally, the resulting gas, liquid, and solid products are collected and separated for further use.
Pyrolysis is not a single, fixed procedure but a controlled decomposition process. The "right" procedure depends entirely on your end goal, as adjusting the heating rate and duration fundamentally changes whether you produce more valuable liquid fuel or more stable solid carbon.

The Core Principle: Heating Without Burning
To understand the procedure, you must first understand the core principle. Pyrolysis is fundamentally different from incineration or burning.
What is Thermochemical Decomposition?
Pyrolysis uses high heat to break the long, complex chemical bonds within a material. This breaks it down into smaller, simpler, and often more valuable molecules.
The process does not destroy the material's chemical energy; it simply rearranges it into different forms.
The Critical Role of an Oxygen-Free Environment
This decomposition happens inside a sealed reactor with no oxygen present. This is the most critical element of the process.
Without oxygen, the material cannot combust (burn). Instead of turning into ash and smoke, it deconstructs into a liquid (bio-oil), a solid (bio-char), and a gas (syngas).
A Step-by-Step Pyrolysis Workflow
While specific steps vary based on the feedstock (the input material), the general workflow follows a clear, logical sequence.
Stage 1: Feedstock Preparation
Before entering the reactor, the raw material must be prepared. This typically involves shredding or grinding to increase surface area and ensure even heating.
The material is also dried to remove moisture, which can hinder the process efficiency. Finally, any non-target materials (like metals mixed in with plastic waste) are separated out.
Stage 2: The Pyrolysis Reactor
This is the heart of the operation. The prepared feedstock is fed into the reactor, which is then sealed and purged of oxygen.
Heat is applied, and the material begins to decompose. Vapors and gases are produced and routed out of the reactor, leaving the solid carbon material (bio-char) behind.
Stage 3: Product Collection and Separation
The hot gas stream exiting the reactor is directed into a condensation unit. Here, the condensable vapors cool and turn into a liquid known as pyrolysis oil or bio-oil.
The remaining non-condensable gases (syngas) are collected separately. The solid bio-char is removed from the bottom of the reactor after the cycle is complete.
Stage 4: Post-Processing (Optional)
Depending on the desired quality, the outputs may be upgraded. The bio-oil, for instance, can undergo distillation and purification to be refined into a higher-grade fuel. The syngas can also be cleaned for use in power generation.
Understanding the Trade-offs: Slow vs. Fast Pyrolysis
The outputs of pyrolysis are not fixed. By controlling the process parameters, you can choose which product you want to maximize. The primary distinction is between slow and fast pyrolysis.
Slow Pyrolysis: Maximizing Solid Bio-char
This method involves heating the material at a lower temperature over a longer period, sometimes for several hours.
This slower process encourages the formation of carbon structures, resulting in a high yield of bio-char. It is the preferred method when the primary goal is to produce a stable, solid carbon product for applications like soil amendment.
Fast Pyrolysis: Maximizing Liquid Bio-oil
This is the most common approach for fuel production. The material is heated very rapidly to a high temperature, with the entire reaction taking just a few seconds.
These conditions "crack" the material into condensable vapors, maximizing the yield of bio-oil (often up to 60% by weight). Syngas and bio-char are produced as co-products.
The Energy Cost
A critical trade-off for any pyrolysis operation is its energy requirement. Reaching and maintaining the high temperatures inside the reactor is an energy-intensive process. Efficient system design and the potential to use the produced syngas to help power the operation are key to economic viability.
Selecting the Right Process for Your Goal
The choice of pyrolysis procedure should be driven by a clear objective. Consider what final product holds the most value for your application.
- If your primary focus is creating valuable liquid fuels (bio-oil): You must use fast pyrolysis to maximize the liquid yield.
- If your primary focus is producing stable solid carbon (bio-char): You should use slow pyrolysis to ensure the highest possible char output.
- If your primary focus is waste volume reduction: Either method is effective, but your decision should be based on which co-product—oil or char—has a more valuable end market for you.
By understanding these principles, you can select and fine-tune the pyrolysis procedure to effectively transform low-value materials into valuable resources.
Summary Table:
| Stage | Key Action | Primary Output |
|---|---|---|
| 1. Preparation | Shredding, Drying, Sorting | Prepared Feedstock |
| 2. Pyrolysis Reactor | Heating in Oxygen-Free Environment | Vapors/Gases & Solid Char |
| 3. Collection | Condensation & Separation | Bio-Oil, Syngas, Bio-Char |
| 4. Post-Processing (Optional) | Distillation, Purification | Upgraded, Higher-Value Products |
Ready to transform your biomass or plastic waste into valuable resources?
The right pyrolysis procedure is key to maximizing your output of bio-oil, bio-char, or syngas. KINTEK specializes in lab-scale pyrolysis equipment and consumables, providing the precise control needed to optimize your process for your specific goals.
Let our experts help you:
- Select the right reactor setup for slow or fast pyrolysis.
- Achieve precise temperature control to target your desired product yield.
- Scale your process efficiently from R&D to production.
Contact our team today to discuss your project and discover how KINTEK's solutions can power your pyrolysis success.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
People Also Ask
- What is safety in pyrolysis process? Managing Extreme Heat and Flammable Products
- What is the use of pyrolysis product? Convert Waste into Fuel, Biochar & Syngas
- What is the efficiency of pyrolysis? Unlocking the True Performance of Your Pyrolysis Process
- What are the advantages of spray pyrolysis? Achieve Cost-Effective, Scalable Thin Film Production
- Why is a vacuum reactor with a rotary drum required for applying oxide coatings to iron powder? Achieve Pure Uniformity
- What is the biochar in pyrolysis reaction? Unlocking Its Role in Soil Enhancement and Carbon Sequestration
- What fuel does a rotary furnace use? Maximize Process Efficiency with Versatile Fuel Options
- What is a calcination furnace? A Guide to High-Temperature Chemical Transformation



















