At its core, Chemical Vapor Deposition (CVD) is a process that uses chemical reactions to create a high-performance thin film on a surface. The process involves placing a substrate in a reaction chamber, introducing specific gases called precursors, and then using heat to trigger a chemical reaction on the substrate's surface, which leaves behind a solid coating.
The fundamental concept to grasp is that CVD is not a physical coating process like painting or plating. It is a thermochemical process where a new, solid material is synthesized directly onto a surface from gaseous reactants.

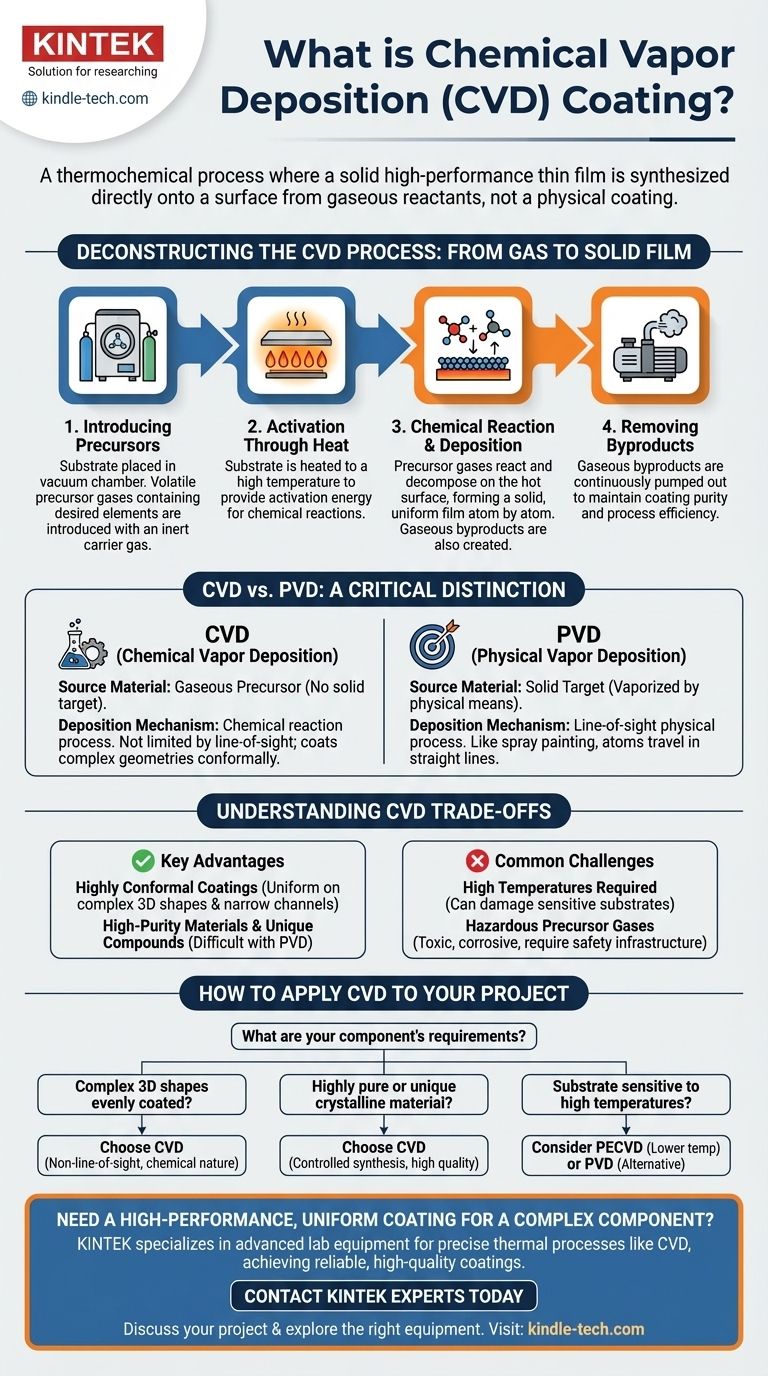
Deconstructing the CVD Process
To truly understand CVD, we must look beyond a simple list of steps and examine the principles at work. The entire process is a carefully controlled chemical reaction designed to build a film one layer of atoms at a time.
Step 1: Introducing the Precursors
The process begins by placing the object to be coated, known as the substrate, inside a vacuum chamber.
Once sealed, a precise mixture of gases is introduced. These aren't just any gases; they are volatile precursors, which are compounds specifically chosen because they contain the elements we want to deposit.
An inert carrier gas is also used to transport the precursors and stabilize the environment inside the chamber.
Step 2: Activation Through Heat
The key activator for the CVD process is thermal energy. The substrate is heated to a specific, often very high, temperature.
This heat is not meant to melt anything. Its sole purpose is to provide the activation energy required for the precursor gases to react and decompose when they come into contact with the hot surface.
Step 3: The Chemical Reaction and Deposition
This is the heart of the process. As the precursor gases flow over the heated substrate, the thermal energy causes them to break apart in a controlled chemical reaction.
The desired atoms from the precursor gas bond to the substrate's surface, beginning to form a thin, uniform film. The other elements from the precursor gas form new, gaseous compounds called byproducts.
This happens across the entire surface of the substrate, allowing CVD to coat complex shapes with exceptional uniformity.
Step 4: Removing the Byproducts
As the solid film builds up on the substrate, the gaseous byproducts of the reaction must be removed.
A vacuum system continuously pumps these volatile byproducts out of the chamber. This prevents them from contaminating the film and ensures the deposition reaction can continue efficiently.
A Critical Distinction: CVD vs. PVD
It's common to confuse Chemical Vapor Deposition (CVD) with Physical Vapor Deposition (PVD), but they operate on fundamentally different principles.
The Source Material
In PVD, the coating material starts as a solid target. This solid is then vaporized into a gas using physical means like sputtering or evaporation.
In CVD, the coating material starts as a gaseous precursor. There is no solid target that gets vaporized inside the chamber.
The Deposition Mechanism
PVD is largely a line-of-sight physical process, much like spray painting. The vaporized atoms travel in a straight line from the source to the substrate.
CVD is a chemical reaction process. Because it relies on gases reacting on a hot surface, it is not limited by line-of-sight and can conformally coat highly complex and intricate geometries.
Understanding the Trade-offs of CVD
No single process is perfect for every application. Understanding the advantages and challenges of CVD is critical for making an informed decision.
Key Advantages
The primary advantage of CVD is its ability to produce highly conformal coatings. It can uniformly coat the inside of long, narrow channels and complex 3D structures where a physical process would fail.
CVD also allows for the creation of very high-purity materials and unique compounds that would be difficult to produce as a solid target for PVD.
Common Challenges
The biggest challenge is often the high temperature required. These temperatures can damage or alter certain substrate materials, limiting the range of applications.
Furthermore, the precursor gases used can be highly toxic, corrosive, or expensive, requiring significant investment in safety and handling infrastructure.
How to Apply This to Your Project
Choosing the right coating technology depends entirely on the requirements of your component and its intended function.
- If your primary focus is coating complex 3D shapes evenly: CVD is often the superior choice due to its non-line-of-sight, chemical reaction-based nature.
- If you need to deposit a highly pure or unique crystalline material: The controlled synthesis possible with CVD allows for exceptional material quality and composition.
- If your substrate is sensitive to high temperatures: You must investigate lower-temperature CVD variants (like PECVD) or consider PVD as a more suitable alternative.
Understanding the fundamental mechanism of how a coating is formed is the key to selecting the right process for your specific goal.
Summary Table:
| CVD Process Step | Key Action | Purpose |
|---|---|---|
| 1. Precursor Introduction | Introduce specific gases into a vacuum chamber | Provide the chemical elements for the coating |
| 2. Thermal Activation | Heat the substrate to a high temperature | Supply energy for the chemical reaction to occur |
| 3. Reaction & Deposition | Precursors react on the hot substrate surface | Forms a solid, uniform thin film atom by atom |
| 4. Byproduct Removal | Pump gaseous byproducts out of the chamber | Maintains coating purity and process efficiency |
Need a high-performance, uniform coating for a complex component?
The CVD process excels at coating intricate 3D geometries with exceptional conformity and material purity. At KINTEK, we specialize in providing advanced lab equipment and consumables for precise thermal processes like CVD. Our solutions help laboratories and manufacturers achieve reliable, high-quality coatings for R&D and production.
Contact our experts today to discuss how CVD can enhance your project and explore the right equipment for your specific application.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- CVD Diamond Cutting Tool Blanks for Precision Machining
People Also Ask
- What is microwave plasma CVD? A Guide to High-Purity Diamond and Material Synthesis
- What are the advantages of using HFCVD for BDD electrodes? Scaling Industrial Diamond Production Efficiently
- How does a Hot Filament Chemical Vapor Deposition (HFCVD) reactor function? Expert Guide to Diamond Film Fabrication
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- How is something diamond coated? A Guide to CVD Growth vs. Plating Methods



















