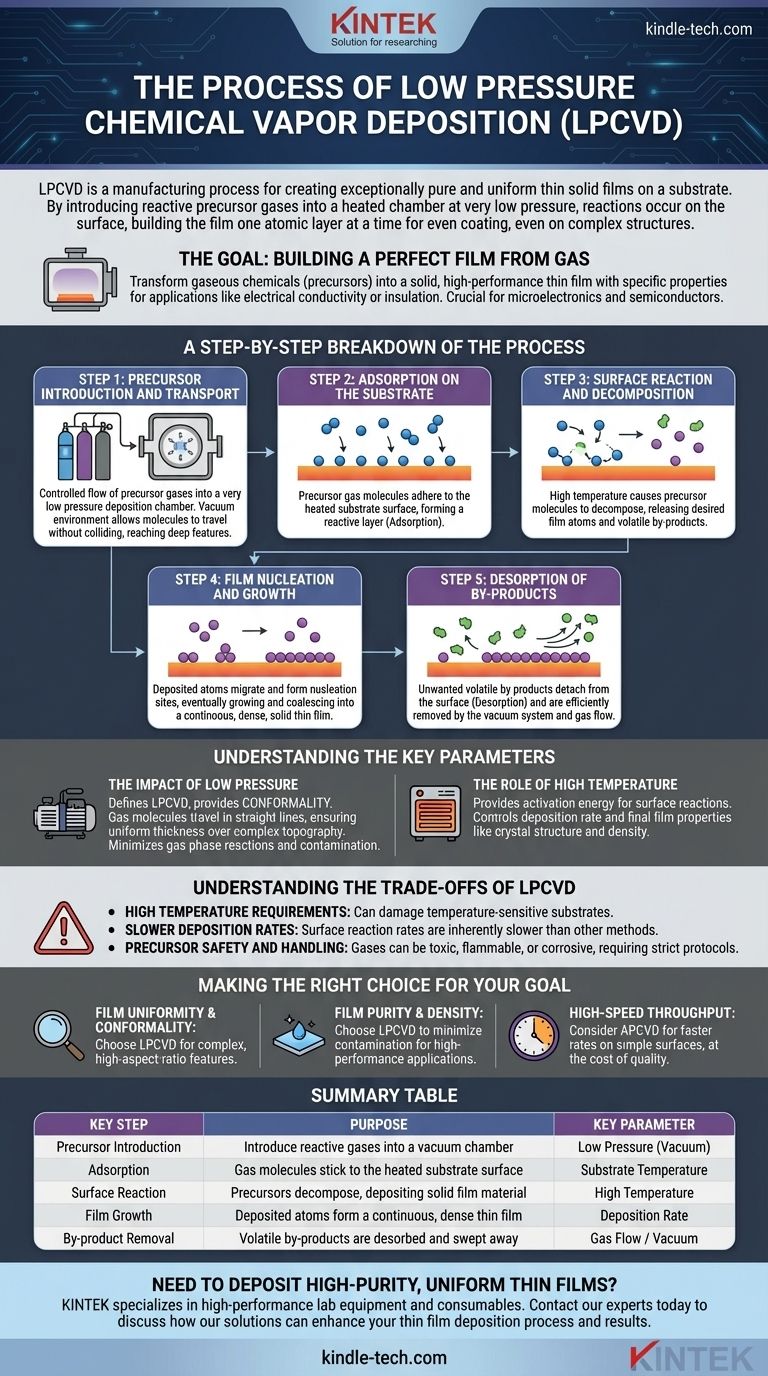
At its core, low pressure chemical vapor deposition (LPCVD) is a manufacturing process used to create exceptionally pure and uniform thin solid films on a substrate. It achieves this by introducing reactive precursor gases into a heated chamber at a very low pressure. The heat causes these gases to decompose and react on the substrate's surface, building the film one atomic layer at a time, while the low pressure ensures the gases coat the surface evenly.
The fundamental advantage of LPCVD isn't just about creating a thin film; it's about achieving unparalleled control. By significantly reducing the chamber pressure, the process forces chemical reactions to occur on the substrate's surface rather than in the gas, leading to exceptionally uniform and pure films even on complex 3D structures.

The Goal: Building a Perfect Film from Gas
The ultimate objective of LPCVD is to transform gaseous chemicals, known as precursors, into a solid, high-performance thin film with specific, desired properties. This film becomes an integral part of the final component, providing characteristics like electrical conductivity, insulation, or wear resistance.
LPCVD is a dominant process in manufacturing microelectronics and semiconductors, where the quality and uniformity of these thin layers are absolutely critical for device performance.
A Step-by-Step Breakdown of the Process
While it happens on a microscopic scale, the LPCVD process follows a clear sequence of physical and chemical events. Each step is precisely controlled to ensure the final film meets specifications.
Step 1: Precursor Introduction and Transport
The process begins by feeding a controlled flow of one or more precursor gases into the deposition chamber. The chamber is kept at a very low pressure, often thousands of times lower than the atmosphere.
This vacuum environment is critical. It allows the gas molecules to travel long distances without colliding, ensuring they reach all areas of the substrate, including deep trenches or complex features.
Step 2: Adsorption on the Substrate
When the precursor gas molecules arrive at the heated substrate, they lose energy and temporarily "stick" to the surface. This physical process is known as adsorption.
The substrate surface is now covered in a layer of reactive molecules, ready for the next stage.
Step 3: Surface Reaction and Decomposition
The high temperature of the substrate provides the thermal energy needed to break the chemical bonds within the adsorbed precursor molecules. This is the central chemical reaction of the process.
The precursors decompose, leaving behind the desired atoms for the film and creating other volatile chemical by-products.
Step 4: Film Nucleation and Growth
The deposited atoms are not initially a uniform film. They migrate across the substrate surface until they find stable "nucleation sites" and begin to form tiny islands of the new material.
These islands grow and coalesce, eventually forming a continuous, dense, and solid thin film on the substrate.
Step 5: Desorption of By-products
The unwanted volatile by-products from the chemical reaction (Step 3) must be removed. These molecules detach from the surface in a process called desorption.
A continuous gas flow through the chamber, maintained by the vacuum system, efficiently sweeps these by-products away, preventing them from being incorporated into the film as impurities.
Understanding the Key Parameters
The success of LPCVD hinges on the precise control of two main variables: pressure and temperature.
The Impact of Low Pressure
Low pressure is what defines LPCVD and gives it its primary advantage: conformality. Because gas molecules can travel in straight lines to the surface, the process is not limited by diffusion. This allows it to deposit a film of perfectly uniform thickness over highly complex and irregular topographies.
Furthermore, by reducing the density of gas molecules, low pressure minimizes unwanted chemical reactions in the gas phase, which would otherwise form particles that contaminate the film.
The Role of High Temperature
Temperature is the engine of the process. It provides the activation energy required to initiate the chemical reactions on the substrate surface.
Controlling the temperature allows engineers to control the deposition rate and influence the film's final properties, such as its crystal structure and density.
Understanding the Trade-offs of LPCVD
While powerful, LPCVD is not the solution for every application. Its primary limitations are a direct consequence of its strengths.
High Temperature Requirements
LPCVD typically operates at high temperatures (often >600°C), which can damage or alter temperature-sensitive substrates like plastics or certain metal layers. This limits the range of materials it can be used with.
Slower Deposition Rates
The process is inherently controlled by surface reaction rates, which are often slower than the mass-transport-limited rates of higher-pressure techniques. This makes LPCVD a relatively slow process, trading speed for superior film quality.
Precursor Safety and Handling
The gases used as precursors in LPCVD can be highly toxic, flammable, or corrosive. This necessitates sophisticated and expensive safety protocols and gas handling systems.
Making the Right Choice for Your Goal
Selecting a deposition technique depends entirely on the required outcome. LPCVD is a high-precision tool for demanding applications.
- If your primary focus is film uniformity and conformality: LPCVD is the superior choice for coating complex, high-aspect-ratio features found in modern microelectronics.
- If your primary focus is film purity and density: The low-pressure environment minimizes particle contamination, making LPCVD ideal for high-performance optical and electronic applications.
- If your primary focus is high-speed throughput on simple, flat surfaces: You might consider alternatives like Atmospheric Pressure CVD (APCVD), which offers faster deposition rates at the cost of film quality.
Ultimately, mastering the LPCVD process is about leveraging its precise control over the deposition environment to build materials with unparalleled quality.
Summary Table:
| Key Step | Purpose | Key Parameter |
|---|---|---|
| Precursor Introduction | Introduce reactive gases into a vacuum chamber | Low Pressure (Vacuum) |
| Adsorption | Gas molecules stick to the heated substrate surface | Substrate Temperature |
| Surface Reaction | Precursors decompose, depositing solid film material | High Temperature |
| Film Growth | Deposited atoms form a continuous, dense thin film | Deposition Rate |
| By-product Removal | Volatile by-products are desorbed and swept away | Gas Flow / Vacuum |
Need to deposit high-purity, uniform thin films for your semiconductor or advanced materials project?
The LPCVD process is a cornerstone of high-precision manufacturing, but it requires expert knowledge and reliable equipment to execute successfully. KINTEK specializes in providing high-performance lab equipment and consumables for demanding laboratory needs. Our expertise can help you leverage LPCVD's advantages—exceptional conformality and film purity—for your most critical applications.
Contact our experts today to discuss how our solutions can enhance your thin film deposition process and results.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings