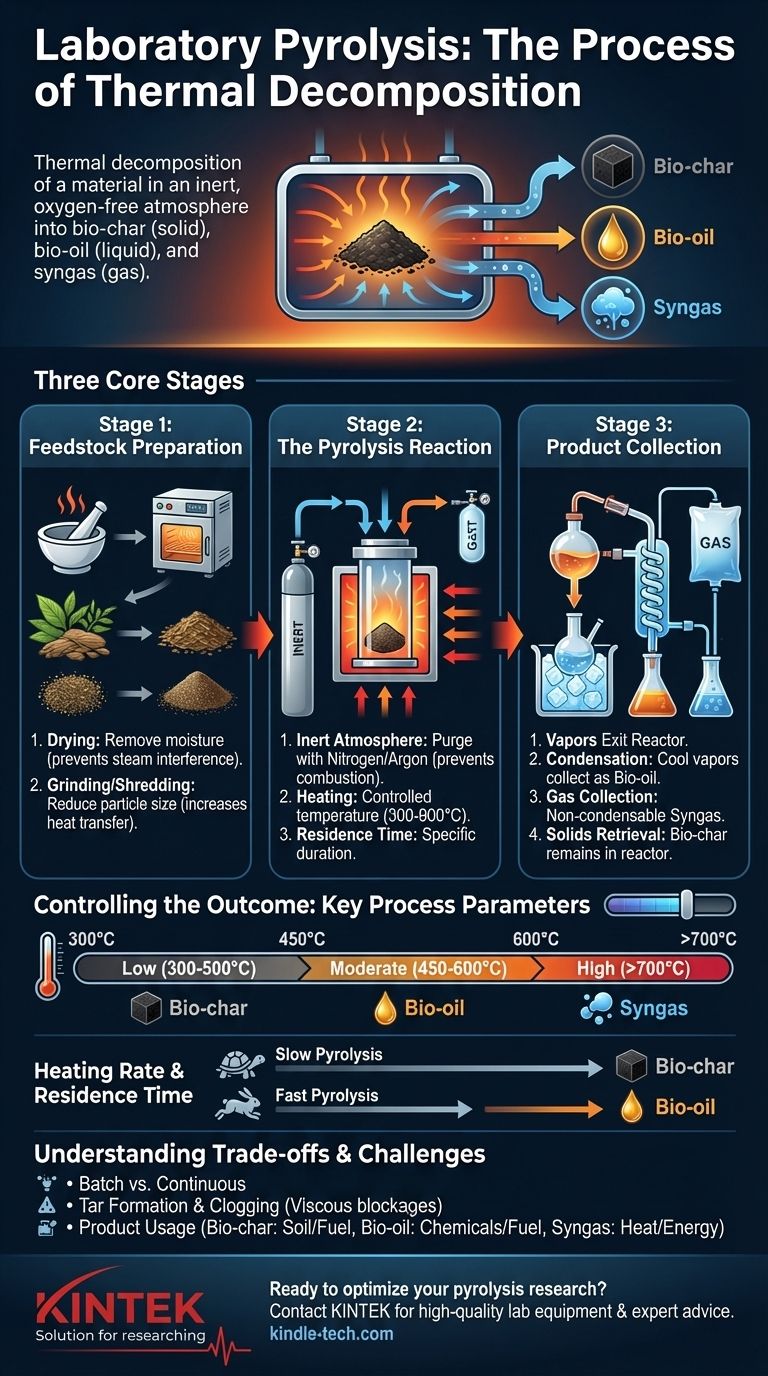
In the laboratory, the process of pyrolysis involves the thermal decomposition of a material by heating it to a high temperature in an inert, oxygen-free atmosphere. This controlled heating breaks down the material into a solid residue (bio-char), a liquid condensate (bio-oil), and a non-condensable gas (syngas). The precise setup and parameters are adjusted to target the desired output product.
Pyrolysis is fundamentally a process of controlled thermal breakdown. The key to success is not just heating the material, but meticulously managing the temperature, heating rate, and atmosphere to prevent combustion and steer the chemical reactions toward producing either solid char, liquid oil, or gas.

The Three Core Stages of Laboratory Pyrolysis
A typical lab-scale pyrolysis experiment can be broken down into three distinct stages, from preparing the initial sample to collecting the final products.
Stage 1: Feedstock Preparation
Before the reaction can begin, the raw material, or feedstock, must be properly prepared. This is a critical step that ensures consistent and repeatable results.
The material is typically dried in an oven to remove moisture. Water can turn to steam during pyrolysis, interfering with the reaction and altering the pressure and product composition.
The dried feedstock is then often ground or shredded into a uniform, small particle size. This increases the surface area, promoting more efficient and even heat transfer throughout the material.
Stage 2: The Pyrolysis Reaction
This is the heart of the process, where the thermal decomposition occurs inside a specialized piece of equipment called a reactor.
A measured amount of the prepared feedstock is placed inside the reactor, which is often a quartz or stainless steel tube. The reactor is then sealed and purged with an inert gas, such as nitrogen or argon, to remove all oxygen and prevent combustion.
The reactor is placed inside a furnace and heated to a specific target temperature, which can range from 300°C to over 900°C. It is held at this temperature for a specific duration known as the residence time.
Stage 3: Product Collection and Separation
As the feedstock decomposes, it releases hot gases and vapors that exit the reactor. These products must be separated and collected.
The hot vapor stream is first passed through a series of condensers, often cooled in an ice bath. This causes the condensable vapors to cool and turn into a liquid, known as pyrolysis oil or bio-oil, which is collected in flasks.
The remaining gases that do not condense are called non-condensable gases or syngas. These can be collected in a gas bag for later analysis or directed to a gas chromatograph or flare.
The solid, carbon-rich material left behind in the reactor after the process is complete is the bio-char, sometimes referred to as coke. It is collected once the reactor has cooled down.
Controlling the Outcome: Key Process Parameters
The ratio of char, oil, and gas produced is not random; it is dictated by the process conditions. By manipulating these key variables, you can favor the production of one product over another.
Temperature
Temperature is the most dominant factor. Lower temperatures (300-500°C) tend to favor the production of solid bio-char, while moderate temperatures (450-600°C) are optimal for maximizing the liquid bio-oil yield. Extremely high temperatures (>700°C) crack the larger molecules further, maximizing gas production.
Heating Rate and Residence Time
The speed at which the material is heated (heating rate) and how long it stays at the peak temperature (residence time) are also critical.
Slow pyrolysis involves a low heating rate and long residence time (hours). This process breaks down the material slowly, maximizing the yield of stable, solid bio-char.
Fast pyrolysis uses a very high heating rate and a very short residence time (a few seconds). This quickly vaporizes the material and rapidly quenches the vapors, which is the ideal method for maximizing the yield of liquid bio-oil.
Understanding the Trade-offs and Challenges
While the principles are straightforward, executing pyrolysis effectively requires understanding its inherent complexities and limitations.
Lab-Scale Batch vs. Industrial Continuous Process
Most laboratory setups use a batch process, where one sample is processed at a time. This offers excellent control for research but has very low throughput.
Industrial systems often use a continuous process, where feedstock is constantly fed into the reactor via mechanisms like a screw feeder. This allows for high throughput but introduces complex engineering challenges related to sealing, heat transfer, and material flow.
Tar Formation and Clogging
One of the most common practical challenges in pyrolysis is the formation of tars. These are thick, viscous organic compounds that can condense in colder parts of the system, causing blockages in tubing and fouling equipment. Managing system temperatures is key to preventing this.
Product Usage and Economics
The resulting products have different uses. Bio-char can be used to improve soil or as a solid fuel. Bio-oil can be a source for chemicals or refined into liquid fuels, though it is often acidic and unstable. The syngas is typically low in energy value but is often burned on-site to provide the heat needed to run the pyrolysis process itself, creating a partially self-sustaining system.
Making the Right Choice for Your Goal
Your experimental design should be dictated by your desired primary output.
- If your primary focus is producing bio-char: Use slow pyrolysis with a slow heating rate, a moderate peak temperature (~400°C), and a long residence time.
- If your primary focus is producing bio-oil: Use fast pyrolysis with a rapid heating rate, a moderate peak temperature (~500°C), and a very short vapor residence time followed by rapid quenching.
- If your primary focus is producing syngas: Use very high temperatures (>700°C) with a longer residence time to ensure complete thermal cracking of the vapors into simple gas molecules.
By understanding these core principles, you can effectively design and execute a pyrolysis process to meet your specific research or production objective.
Summary Table:
| Process Parameter | Effect on Product Yield |
|---|---|
| Low Temperature (300-500°C) | Maximizes solid Bio-char |
| Moderate Temperature (450-600°C) | Maximizes liquid Bio-oil |
| High Temperature (>700°C) | Maximizes Syngas |
| Slow Heating Rate / Long Residence Time | Favors Bio-char production (Slow Pyrolysis) |
| Fast Heating Rate / Short Residence Time | Favors Bio-oil production (Fast Pyrolysis) |
Ready to optimize your pyrolysis research?
KINTEK specializes in high-quality lab equipment and consumables for precise thermal decomposition processes. Whether you need robust reactors, efficient condensers, or expert advice to configure your system for maximum bio-char, bio-oil, or syngas yield, we have the solutions to enhance your lab's capabilities and accelerate your results.
Contact our experts today to discuss your specific pyrolysis application and discover the right equipment for your laboratory needs.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- What is the range of pyrolysis? Master Temperature Control for Optimal Bio-Product Yields
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies
- At what temperature is conventional pyrolysis done? Unlock the Right Temperature for Your Desired Product
- Why are high temperatures required when sintering stainless steels? Unlock Pure, High-Density Results



















