At its core, slow pyrolysis is a thermal decomposition process that heats organic materials, such as biomass or plastic, in a completely oxygen-free or oxygen-limited environment. Unlike faster methods, its defining feature is a very slow, controlled heating rate. This deliberate pace is specifically designed to maximize the conversion of the feedstock into a stable, carbon-rich solid known as biochar.
Slow pyrolysis is not just a method of heating; it is a strategic choice. By precisely controlling the heating rate and temperature, the process intentionally favors the creation of a solid char product over the generation of liquids or gases, effectively locking carbon into a stable, useful form.

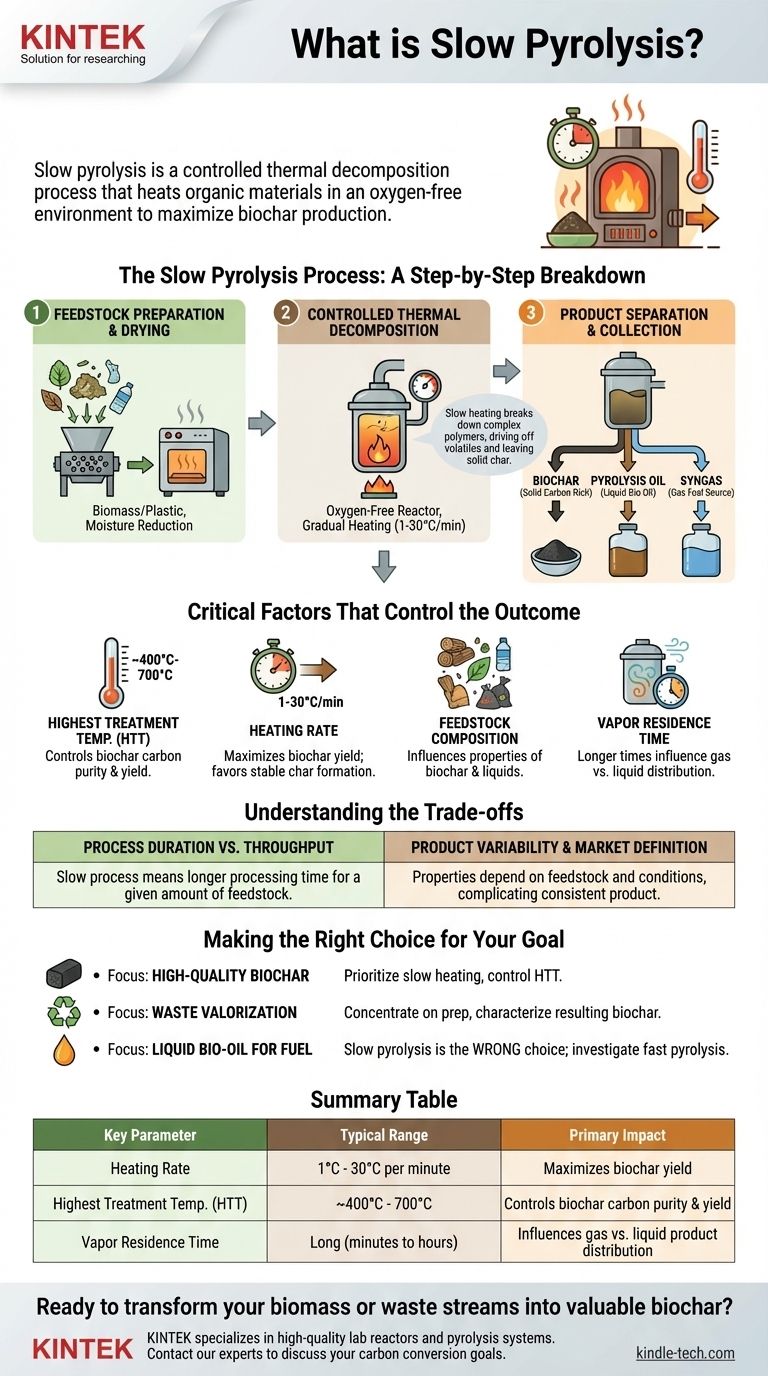
The Slow Pyrolysis Process: A Step-by-Step Breakdown
The process can be understood as a sequence of three fundamental stages, from preparing the raw material to collecting the final, value-added products.
Step 1: Feedstock Preparation and Drying
Before the thermal reaction can begin, the raw material, or feedstock, must be properly prepared. This almost always involves drying the material to significantly reduce its moisture content.
For many feedstocks, this stage may also include shredding to create a uniform particle size or preprocessing to separate non-target materials, ensuring an efficient and consistent reaction.
Step 2: Controlled Thermal Decomposition
This is the heart of the process. The dried feedstock is loaded into a reactor, which is then sealed to create an oxygen-free environment.
Heat is applied gradually, with typical heating rates between 1°C and 30°C per minute. This slow heating breaks down the complex organic polymers in the feedstock, driving off volatile compounds as gases and liquids while leaving behind a solid, carbon-dense structure.
Step 3: Product Separation and Collection
As the reaction completes, the resulting products are separated. The primary product, biochar (or biocoal), is the solid material that remains in the reactor.
The volatile gases are directed away from the reactor. As they cool, some of these gases condense into a liquid, often called pyrolysis oil, bio-oil, or wood vinegar. The remaining non-condensable gases, known as syngas, are also collected and can be used as a fuel source, sometimes to power the pyrolysis process itself.
Critical Factors That Control the Outcome
The final yield and characteristics of the products are not accidental; they are a direct result of several key process parameters. Understanding these variables is crucial for engineering a desired result.
Highest Treatment Temperature (HTT)
This is arguably the most influential factor. Higher temperatures (e.g., >500°C) generally lead to a higher-purity carbon in the biochar but can reduce the overall char yield as more material is converted into gas.
Heating Rate
The slow heating rate is the defining feature of this process. It allows time for complex secondary reactions to occur, which favors the formation of stable char structures rather than volatile liquids and gases.
Feedstock Composition
The nature of the starting material heavily influences the final products. A woody biomass will produce a different biochar and liquid than a plastic waste stream would, impacting its chemical composition, porosity, and potential uses.
Vapor Residence Time
This refers to how long the volatile gases remain in the hot zone of the reactor. Longer residence times can lead to secondary cracking, where gas and liquid molecules break down further, potentially increasing the yield of gas at the expense of liquid.
Understanding the Trade-offs
While effective for producing biochar, slow pyrolysis is a process of deliberate compromise that presents certain challenges.
Process Duration vs. Throughput
The primary trade-off is speed. The "slow" nature of the process means that the time required to process a given amount of feedstock is significantly longer than in fast pyrolysis, which can limit the overall throughput of a facility.
Product Variability and Market Definition
The properties of the biochar and bio-oil are highly dependent on both the feedstock and the precise operating conditions. This variability can make it difficult to produce a perfectly consistent product, which in turn complicates efforts to define a clear market and price point.
Making the Right Choice for Your Goal
Your choice of pyrolysis parameters should be dictated by your end goal. Use this as a guide to align the process with your desired outcome.
- If your primary focus is producing high-quality biochar: Prioritize slow heating rates and carefully control the highest treatment temperature to achieve the desired carbon content and stability.
- If your primary focus is waste valorization: Concentrate on effective feedstock preparation and drying, and be prepared to characterize the resulting biochar to find its most suitable application.
- If your primary focus is producing liquid bio-oil for fuel: Slow pyrolysis is the wrong choice; you should investigate fast pyrolysis, which uses rapid heating to maximize liquid yield.
By understanding these core principles, you can leverage slow pyrolysis as a precise tool to transform low-value organic materials into a specific and valuable carbon product.
Summary Table:
| Key Parameter | Typical Range | Primary Impact |
|---|---|---|
| Heating Rate | 1°C - 30°C per minute | Maximizes biochar yield |
| Highest Treatment Temp. (HTT) | ~400°C - 700°C | Controls biochar carbon purity & yield |
| Vapor Residence Time | Long (minutes to hours) | Influences gas vs. liquid product distribution |
Ready to transform your biomass or waste streams into valuable biochar? The precise control required for an effective slow pyrolysis process demands reliable equipment. KINTEK specializes in high-quality lab reactors and pyrolysis systems designed for consistent, scalable results. Contact our experts today to discuss how our solutions can help you achieve your carbon conversion and valorization goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Lab-Scale Vacuum Induction Melting Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What is sputter coating SEM sample preparation? Eliminate Charging for Crisp, Stable Images
- What is the purpose of using an ultrasonic cleaner or dispersion instrument? Boost Photocatalytic CO2 Reduction Efficiency
- What is the primary function of a high-speed magnetic stirrer in the synthesis of Pd-on-Au NPs? Ensure Uniform Diffusion
- What is the difference between cast and sintered parts? Choose the Right Metal Forming Process
- Why is a vacuum distillation system necessary during the synthesis of rosin allyl esters? Protect Product Integrity
- Why Use Controlled Drying for Zr-doped CaO? Preserve Porosity and Prevent Agglomeration
- What are the factors affecting the heat treatment of steel? Master the Process for Superior Material Properties
- What is a heat treatment furnace? Achieve Precise Metallurgical Transformations



















