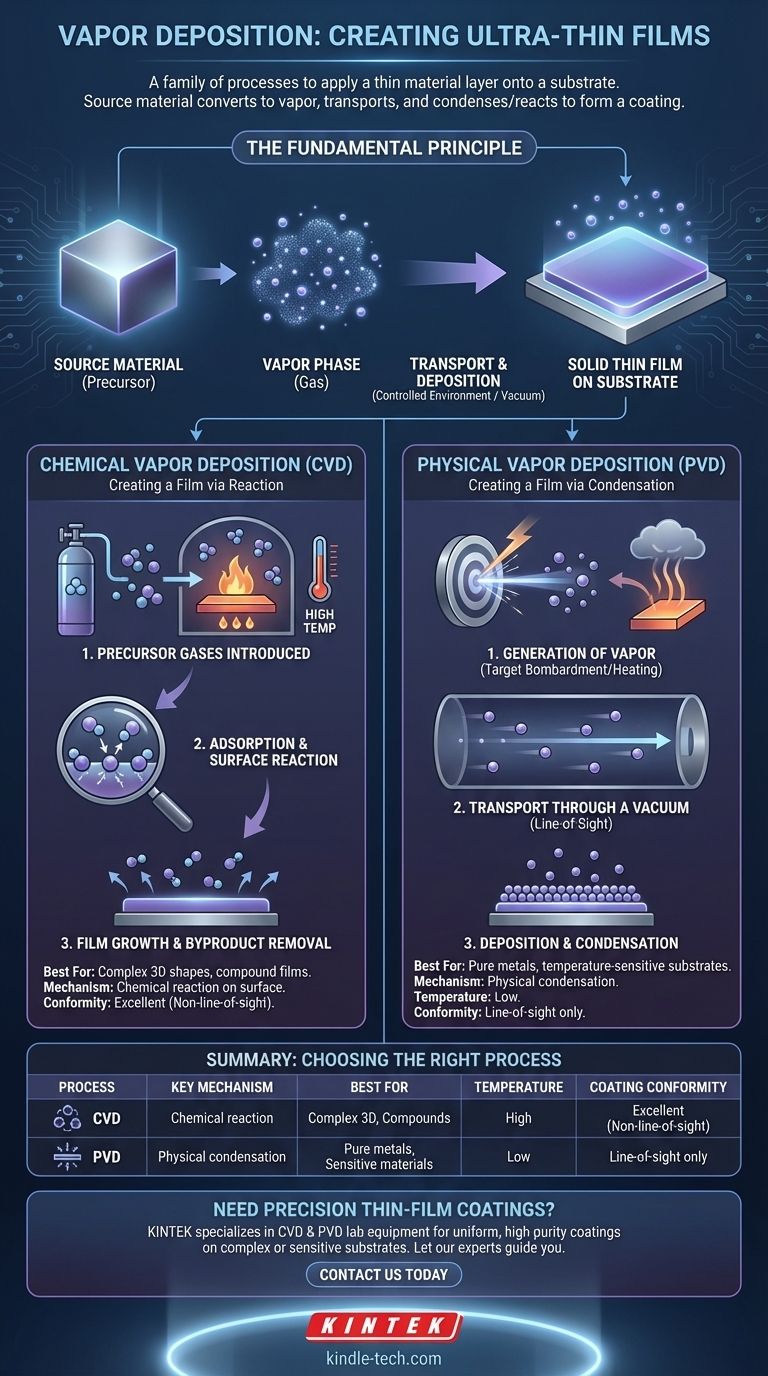
Vapor deposition is a family of processes used to apply an ultra-thin film of material onto a surface, known as a substrate. In all cases, a source material is converted into a gaseous vapor, transported, and then condensed or reacted onto the substrate's surface to form the desired coating. The specific method used determines the properties and quality of the final film.
At its core, vapor deposition is about moving atoms or molecules in a gas phase onto a solid surface to build a new layer. The fundamental distinction between its two main types, Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD), comes down to a simple question: does a chemical reaction create the film, or is it formed by a direct physical state change?

The Fundamental Principle: From Gas to Solid Film
Vapor deposition operates within a controlled environment, typically a vacuum chamber, to ensure purity and precision. This control is what allows for the creation of films that can be just a few atoms thick.
### The Source Material
The process begins with a source material, also known as a precursor. This is the substance you want to deposit as a thin film.
### The Vapor Phase
This source material is converted into a gas. How this happens is the primary difference between the major deposition techniques.
### Transport and Deposition
The vaporized material travels through the chamber and deposits onto the target substrate, which has been cleaned and prepared. This deposition forms a stable, solid thin film on the substrate's surface.
Chemical Vapor Deposition (CVD): Creating a Film via Reaction
In Chemical Vapor Deposition (CVD), the film is not made of the original gas itself. Instead, the gas is a chemical precursor that reacts on the substrate's surface to form an entirely new solid material.
### Step 1: Introduction of Precursor Gases
One or more volatile precursor gases are introduced into the reaction chamber containing the heated substrate. The substrate is intentionally kept at a high temperature to drive the chemical reaction.
### Step 2: Adsorption and Surface Reaction
The gas molecules adsorb (stick) to the hot surface of the substrate. The thermal energy from the substrate causes the gases to decompose or react with each other.
### Step 3: Film Growth and Byproduct Removal
This chemical reaction forms the desired solid film on the substrate. Gaseous byproducts from the reaction are then desorbed from the surface and transported out of the chamber by the gas flow or vacuum system.
Physical Vapor Deposition (PVD): Creating a Film via Condensation
In Physical Vapor Deposition (PVD), the process is a direct physical transformation. The source material is physically turned into a vapor, which then travels and condenses back into a solid on the substrate, with no chemical reaction occurring.
### Step 1: Generation of Vapor
A solid source material, known as the "target," is bombarded with energy to generate a vapor. This is often done through sputtering (using energetic ions to knock atoms off the target) or thermal evaporation (heating the material until it boils).
### Step 2: Transport Through a Vacuum
The vaporized atoms or molecules travel through a vacuum chamber. Because PVD is typically a "line-of-sight" process, the atoms travel in a straight line from the source target to the substrate.
### Step 3: Deposition and Condensation
When the vaporized atoms strike the cooler substrate, they condense back into a solid state, gradually building up the thin film. The process is akin to steam condensing on a cold mirror.
Understanding the Trade-offs
Choosing between CVD and PVD depends entirely on the material, the shape of the substrate, and the desired properties of the final coating. Neither is universally superior.
### Coating Conformity
CVD excels at creating highly conformal coatings. Because the precursor is a gas that surrounds the substrate, the chemical reaction can occur on all exposed surfaces, even in complex, non-line-of-sight geometries.
PVD is primarily a line-of-sight process. Areas of the substrate that are shadowed from the source target will receive little to no coating, making it less suitable for intricate shapes without complex substrate manipulation.
### Operating Temperature
CVD typically requires a very high substrate temperature to activate and drive the necessary chemical reactions on the surface. This can limit the types of materials that can be used as substrates.
PVD can often be performed at much lower temperatures. This makes it compatible with a wider range of materials, including plastics and other temperature-sensitive substrates.
### Material Purity
PVD can deposit extremely pure materials, as the film has the same composition as the source target. It is excellent for depositing pure metals, alloys, and certain ceramics.
CVD films can sometimes contain impurities from the precursor gases or incomplete reactions. However, it is uniquely capable of forming compounds that are difficult or impossible to create as a PVD target, such as diamond-like carbon or silicon nitride.
Making the Right Choice for Your Goal
Your application's specific requirements will dictate the most appropriate deposition method.
- If your primary focus is coating complex, 3D shapes uniformly: CVD is the superior choice due to its non-line-of-sight, gas-phase reaction.
- If your primary focus is depositing a highly pure metal or alloy onto a temperature-sensitive substrate: PVD offers precise control over film composition at lower process temperatures.
- If your primary focus is creating a specific chemical compound like silicon dioxide or diamond-like carbon: CVD is often the only practical method, as it builds the compound directly on the surface via chemical reaction.
Understanding the fundamental difference between a chemical reaction and a physical state change is the key to selecting the correct vapor deposition process for your needs.
Summary Table:
| Process | Key Mechanism | Best For | Temperature | Coating Conformity |
|---|---|---|---|---|
| Chemical Vapor Deposition (CVD) | Chemical reaction on substrate surface | Complex 3D shapes, compound films | High temperature | Excellent (non-line-of-sight) |
| Physical Vapor Deposition (PVD) | Physical condensation of vapor | Pure metals, temperature-sensitive substrates | Low temperature | Line-of-sight only |
Need Precision Thin-Film Coatings for Your Laboratory?
KINTEK specializes in lab equipment and consumables for vapor deposition processes. Whether you require CVD systems for complex geometries or PVD equipment for pure metal coatings, our solutions deliver superior film quality and process control.
We help laboratories:
- Achieve uniform coatings on intricate substrates
- Deposit high-purity metals and alloys
- Work with temperature-sensitive materials
- Create specialized compound films
Let our experts guide you to the right deposition technology for your specific application.
Contact us today to discuss your project requirements and discover how KINTEK can enhance your thin-film research and production capabilities.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the advantages and disadvantages of sputtering? Balancing Film Quality, Speed, and Cost
- What are the advantages of MCVD? Achieve Unmatched Purity and Precision in Optical Fiber Fabrication
- What are the advantages of chemical Vapour deposition method in CNT? Achieve Unmatched Control for Your Nanotube Synthesis
- How are reactants introduced into the reaction chamber during a CVD process? Mastering Precursor Delivery Systems
- What is CVD in coating? A Guide to High-Performance Chemical Vapor Deposition
- What is the process of CVD reaction? A Step-by-Step Guide to High-Performance Coating
- What is deposition in nanotechnology? Build High-Performance Materials Atom by Atom
- What function does high-purity nitrogen gas serve in AACVD? Enhance Your Titanium Dioxide Film Quality Today



















