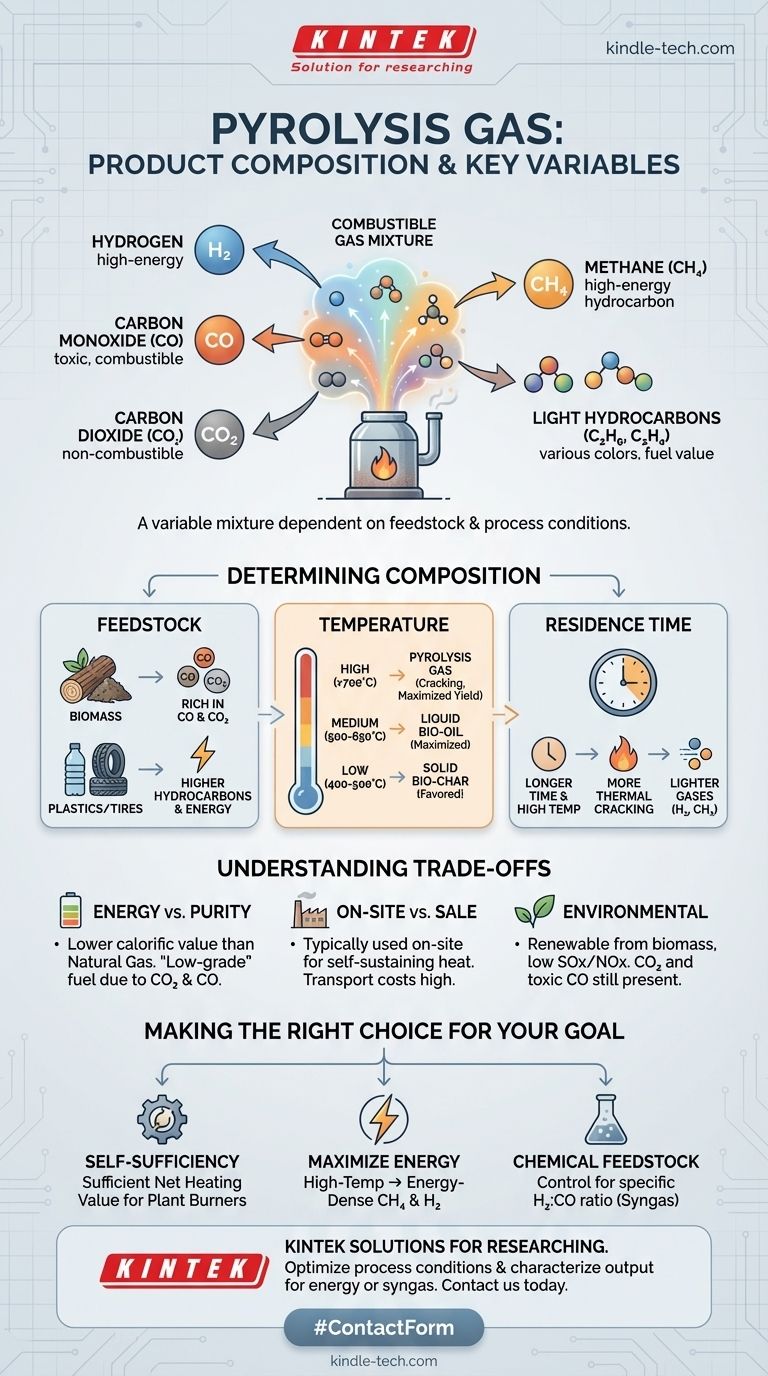
At its core, pyrolysis gas is a combustible mixture primarily composed of hydrogen (H₂), carbon monoxide (CO), carbon dioxide (CO₂), and methane (CH₄). It also contains smaller quantities of other light hydrocarbons like ethane and ethylene. This gas is one of the three primary products of pyrolysis, alongside a liquid (bio-oil) and a solid (bio-char).
The specific composition of pyrolysis gas is not fixed. It is a direct outcome of two key variables: the type of material being processed (the feedstock) and the precise conditions of the pyrolysis reaction, especially temperature. Understanding these factors is crucial to controlling the gas's energy content and ultimate value.

What Determines the Gas Composition?
The ratio of components in pyrolysis gas is highly variable. Engineers and operators manipulate process parameters to achieve a gas composition that is optimized for a specific goal, whether it's maximizing energy production or creating a chemical precursor.
The Role of the Feedstock
The material you start with dictates the final output. The chemical structure of the input feedstock has a direct and significant impact on the resulting gas mixture.
For example, the pyrolysis of biomass (like wood or agricultural waste) typically yields a gas rich in CO and CO₂. In contrast, the pyrolysis of plastics or tires will produce a gas with a higher concentration of valuable hydrocarbons, resulting in a higher overall energy content.
The Impact of Process Temperature
Temperature is the most powerful lever for controlling pyrolysis outputs. As you increase the temperature of the reactor, you fundamentally change which product is favored.
A general rule is that lower temperatures (around 400-500°C) favor the production of the solid bio-char. As temperatures increase into the medium range (500-650°C), liquid bio-oil production is maximized. At high temperatures (above 700°C), the process "cracks" the larger molecules, maximizing the yield of pyrolysis gas.
The Influence of Residence Time
Residence time—how long the feedstock is exposed to the heat inside the reactor—also plays a role. Longer residence times at high temperatures promote further thermal cracking, breaking down heavier oils and tars into lighter, non-condensable gases like hydrogen and methane.
Understanding the Trade-offs
Pyrolysis gas is a valuable product, but its application is governed by practical and economic trade-offs. Understanding its limitations is as important as knowing its potential.
Energy Content vs. Purity
While pyrolysis gas is a useful fuel, its energy content (calorific value) is generally lower than that of natural gas. This is because it contains significant amounts of non-combustible CO₂ and lower-energy CO alongside high-energy methane and hydrogen.
The presence of these components makes it a "low-grade" or "medium-grade" fuel gas. While perfectly suitable for many industrial heating applications, it would require significant processing and purification to be used as a substitute for pipeline-quality natural gas.
On-site Use vs. External Sale
Due to its variable composition and relatively low energy density, pyrolysis gas is most often used directly on-site. The gas produced is typically recycled to provide the heat needed to run the pyrolysis reactor itself.
This creates a highly efficient, self-sustaining system. The cost and complexity of cleaning, compressing, and transporting the gas for external sale often outweigh the economic benefit, unless the facility is operating at a very large scale.
Environmental Considerations
When sourced from biomass, pyrolysis gas is considered a renewable energy source. Its combustion typically produces very low levels of sulfur oxides (SOx) and nitrogen oxides (NOx), making it a cleaner-burning fuel than many fossil-based alternatives. However, it still produces carbon dioxide upon combustion, and any unburned carbon monoxide is toxic.
Making the Right Choice for Your Goal
The "best" pyrolysis gas composition depends entirely on your end goal. By controlling feedstock and process conditions, you can steer the output to meet your specific needs.
- If your primary focus is process self-sufficiency: The exact gas composition is less critical, as long as it has a sufficient net heating value to fuel the pyrolysis plant's burners.
- If your primary focus is maximizing energy output: You would aim for high-temperature conditions that favor the production of energy-dense methane (CH₄) and hydrogen (H₂).
- If your primary focus is creating chemical feedstocks: You would carefully control conditions to produce a specific ratio of hydrogen to carbon monoxide (H₂:CO), creating a product known as "syngas" for further chemical synthesis.
Ultimately, mastering the variables of the pyrolysis process empowers you to transform diverse waste streams into a tailored and valuable fuel source.
Summary Table:
| Key Component | Typical Role/Characteristic |
|---|---|
| Hydrogen (H₂) | High-energy gas, desirable for fuel value and chemical synthesis. |
| Carbon Monoxide (CO) | Combustible gas, but toxic; a key component of syngas. |
| Methane (CH₄) | High-energy hydrocarbon, increases the gas's calorific value. |
| Carbon Dioxide (CO₂) | Non-combustible gas, dilutes the mixture and lowers energy content. |
| Other Hydrocarbons (C₂H₆, C₂H₄) | Contribute to the overall fuel value of the gas mixture. |
Ready to harness the power of pyrolysis gas in your operations?
The composition of your pyrolysis gas is critical to your project's efficiency and economic viability. At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to analyze feedstocks, optimize process conditions, and accurately characterize your gas output.
Whether you're focused on achieving energy self-sufficiency or producing syngas for chemical feedstocks, our solutions support your R&D and quality control efforts.
Contact us today using the form below to discuss how KINTEK can equip your laboratory for success in pyrolysis and bioenergy research.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Square Lab Press Mold for Laboratory Applications
- Hydraulic Diaphragm Lab Filter Press for Laboratory Filtration
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success


















