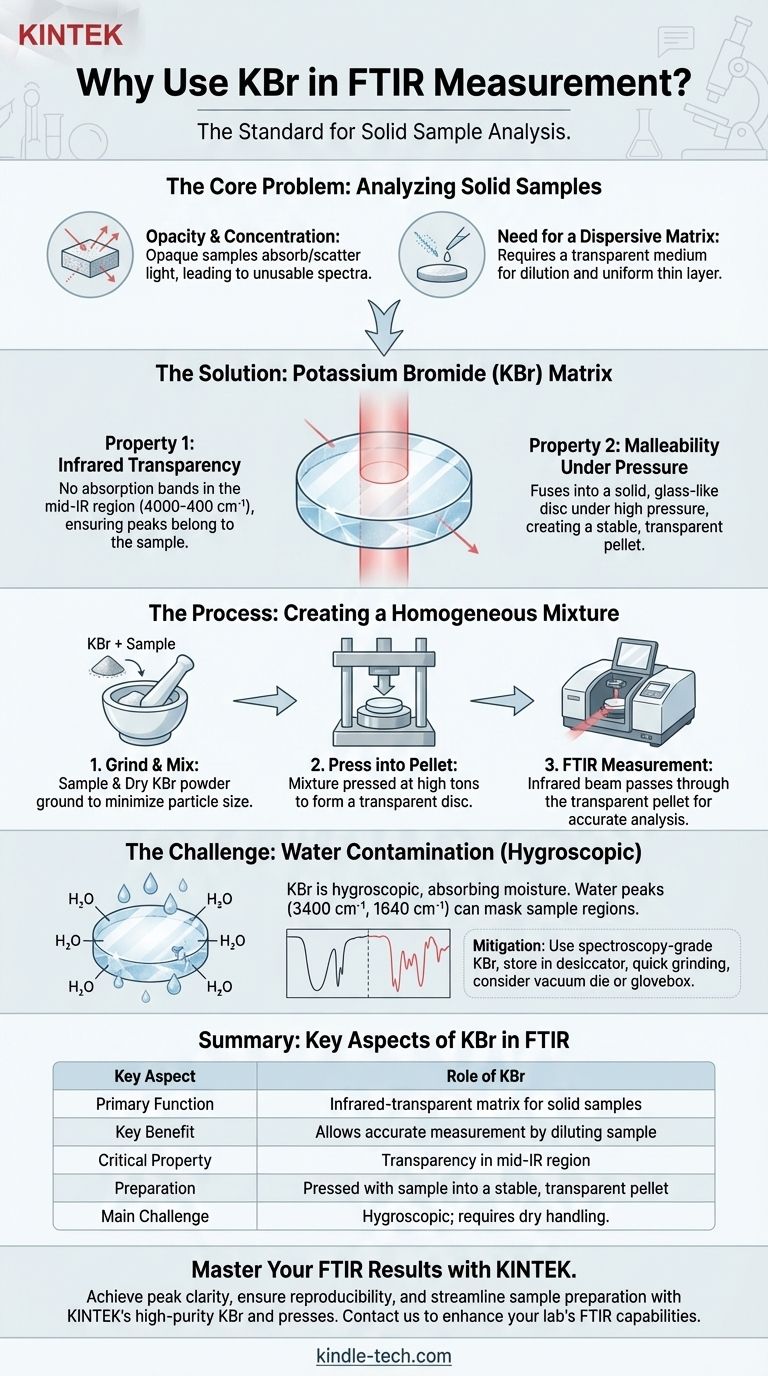
In FTIR spectroscopy, potassium bromide (KBr) is used as an infrared-transparent solid matrix to hold a sample for analysis. Because solid samples are often opaque and too concentrated, they must be finely dispersed in a medium that does not interfere with the measurement. KBr is the ideal choice because it is transparent to infrared light and can be pressed with the sample into a thin, transparent pellet.
The core challenge in analyzing solid samples with FTIR is getting the infrared beam to pass through the material without being completely absorbed or scattered. KBr solves this by acting as a diluent and a physical support, allowing a small, representative amount of the sample to be measured accurately within a transparent medium.

The Core Problem: Analyzing Solid Samples
Most organic and inorganic solids are difficult to analyze directly with transmission FTIR. The KBr pellet method is a classic solution designed to overcome two fundamental obstacles.
The Challenge of Opacity and Concentration
A pure solid powder or crystal is typically too concentrated. It will absorb nearly all the infrared light at its characteristic frequencies, resulting in a spectrum with flat-topped, unusable peaks (a phenomenon known as total absorbance).
Furthermore, light scattering from the particles of a solid sample can distort the spectrum and reduce the signal-to-noise ratio, making interpretation difficult.
The Need for a Dispersive Matrix
To get a useful spectrum, the sample must be diluted and held in a uniform, thin layer. This requires a matrix material that can be mixed with the sample.
This matrix must be transparent in the mid-infrared region (typically 4000-400 cm⁻¹) so that it does not contribute its own absorption bands to the spectrum, which would obscure the signals from the actual sample.
Why Potassium Bromide is the Standard Choice
KBr became the gold standard for this technique due to a unique combination of optical and physical properties.
Property 1: Infrared Transparency
The primary reason for using KBr is its lack of absorption bands in the most analytically useful region of the infrared spectrum. This ensures that every peak observed in the final spectrum belongs to the sample, not the matrix.
Property 2: Malleability Under Pressure
When finely ground and subjected to high pressure (typically several tons in a hydraulic press), KBr powder flows and fuses into a solid, glass-like disc. This process creates a transparent pellet that is mechanically stable and ideal for placing in the spectrometer's sample holder.
The Goal: Creating a Homogeneous Mixture
The preparation involves grinding a tiny amount of sample (typically a 1:100 ratio of sample to KBr) with dry KBr powder. This grinding is critical to reduce sample particle size below the wavelength of the IR light, which minimizes scattering.
This process disperses the sample molecules evenly throughout the KBr matrix, creating what is effectively a solid solution. The resulting pellet contains the sample in a low enough concentration to produce a sharp, well-defined spectrum.
Understanding the Trade-offs: The Challenge of Water
While KBr is an excellent matrix, it has one significant drawback that is the most common source of error in this technique.
The Problem: A Hygroscopic Nature
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong and broad infrared absorption peaks, primarily around 3400 cm⁻¹ (O-H stretching) and 1640 cm⁻¹ (H-O-H bending).
The Impact of Water Contamination
If the KBr used is not perfectly dry, these large water peaks can obscure important sample peaks in those regions. For example, the O-H or N-H stretching regions of a sample can be completely masked by water contamination.
Best Practices for Mitigation
To get a clean spectrum, always use spectroscopy-grade KBr that has been stored in a desiccator or drying oven. Grind the sample and KBr quickly, and in humid environments, consider preparing the pellet inside a glovebox or using a special vacuum die to remove air and moisture during pressing.
Making the Right Choice for Your Goal
Properly preparing a KBr pellet is a fundamental laboratory skill. The level of rigor required depends on your analytical goal.
- If your primary focus is compound identification: Ensure your KBr is dry and aim for a visually transparent pellet to get a clear, interpretable spectrum.
- If your primary focus is quantitative analysis: Precise sample-to-KBr ratios, consistent grinding, and uniform pellet thickness are critical for achieving reproducible results.
- If you are analyzing a sample for hydroxyl (-OH) groups: Using a vacuum die or performing the preparation in a dry glovebox is essential to avoid misleading artifacts from water contamination.
Mastering the KBr pellet technique is a key skill that transforms intractable solid materials into sources of clear, high-quality spectroscopic data.
Summary Table:
| Key Aspect | Role of KBr in FTIR |
|---|---|
| Primary Function | Infrared-transparent matrix for solid samples |
| Key Benefit | Allows accurate measurement by diluting the sample |
| Critical Property | Transparency in the mid-IR region (4000-400 cm⁻¹) |
| Preparation | Pressed with sample into a stable, transparent pellet |
| Main Challenge | Hygroscopic nature; requires dry handling to avoid water peaks |
Master the KBr pellet technique for superior FTIR results.
Are you struggling to get clear, interpretable spectra from your solid samples? KINTEK specializes in providing high-purity, spectroscopy-grade potassium bromide (KBr) and reliable laboratory presses designed specifically for FTIR pellet preparation.
We understand that accurate sample analysis is critical for your research and quality control. Our products help you:
- Achieve peak clarity: Minimize scattering and absorption artifacts with optimal KBr dispersion.
- Ensure reproducibility: Get consistent, reliable results with precise pellet formation.
- Save time and resources: Streamline your sample preparation workflow with our trusted equipment and consumables.
Ready to enhance your lab's FTIR capabilities? Contact our experts today via our Contact Form to find the perfect KBr and pressing solutions for your specific laboratory needs.
Visual Guide
Related Products
- kbr pellet press 2t
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Lab Infrared Press Mold
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What are the applications of hydraulic press hammer like equipment? Unlock Precision Force for Your Manufacturing
- What materials are being analyzed by XRF? Discover Its Versatility for Elemental Analysis
- What is the core function of a laboratory hydraulic press in the production of fuel cell bipolar plates? Expert Guide
- What is the construction of a hydraulic press machine? A Guide to Its Core Systems & Force Generation
- Why is a laboratory hydraulic press used for pre-forming LiFePO4 powders? Enhancing Structural Integrity for CIP
- Why is a hydraulic press required for the lamination process? Essential Steps for Solid-State Battery Assembly
- How is a laboratory hydraulic press used to evaluate the mechanical performance of nano-modified concrete? Expert Guide
- What is the role of a high-pressure rolling system in ceramic MFC chassis? Ensure Uniformity and Peak Performance



















