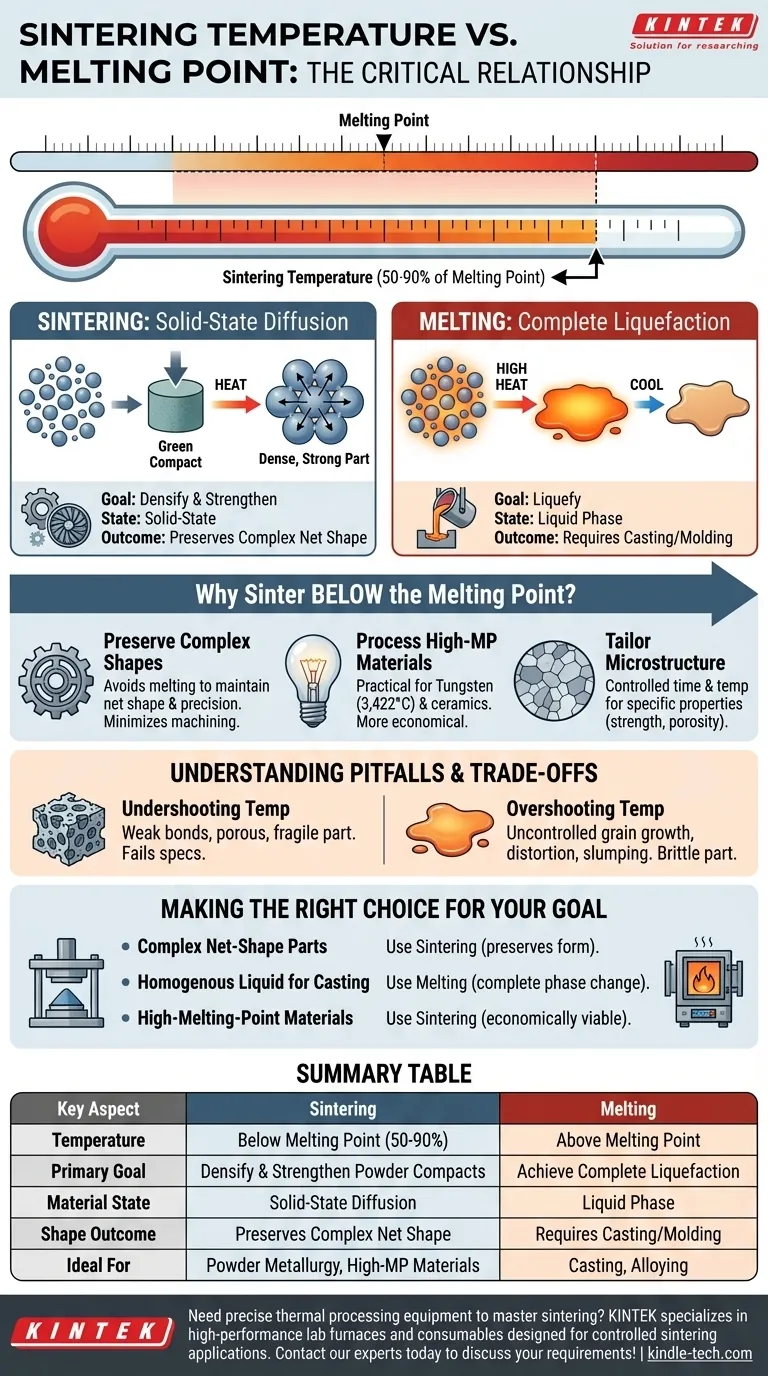
The critical relationship between sintering temperature and a material's melting point is one of a carefully controlled boundary. Sintering is a thermal process that intentionally occurs at a temperature below the melting point, typically between 50% and 90% of the melting temperature. This distinction is not arbitrary; it is the fundamental principle that defines the sintering process and separates it from casting or welding.
The core difference is not just temperature, but mechanism. Melting uses heat to achieve a complete phase change (solid to liquid), while sintering uses heat to energize solid particles, causing them to fuse together through atomic diffusion without ever liquefying.
Sintering vs. Melting: Two Different Thermal Goals
To grasp the relationship, you must understand that these processes are designed to achieve fundamentally different outcomes at the atomic level.
Melting: The Path of Complete Liquefaction
Melting has one simple objective: to heat a material above its melting point until it transforms entirely from a solid into a liquid.
The temperature is raised to overcome the crystalline structure, allowing atoms to move freely. This liquid can then be poured, cast, or mixed.
Sintering: The Path of Solid-State Diffusion
Sintering’s goal is to increase the density and strength of a compacted powder mass. The temperature is raised to a point high enough to energize the atoms within the solid particles.
This energy allows atoms to migrate across the boundaries where particles touch, forming strong metallic or ceramic bonds. The material fuses into a coherent solid mass while remaining in the solid state.
The Melting Point as a Hard Ceiling
For a standard sintering process, the melting point acts as a strict upper limit. Exceeding it would defeat the purpose, causing the precisely shaped powder compact to slump, distort, or turn into a puddle.
The success of sintering relies on finding the "sweet spot"—a temperature hot enough for rapid diffusion but safely below the point of melting.
Why Sinter Below the Melting Point?
Choosing to operate below the melting point is a deliberate engineering decision that provides several key advantages.
Preserving Complex Shapes
Sintering is a core part of powder metallurgy, where powders are first pressed into a "green compact" with a specific, often complex, shape.
By avoiding melting, the process preserves this net shape with high precision, minimizing the need for subsequent machining.
Processing High-Melting-Point Materials
Materials like tungsten (melting point: 3,422°C) and many technical ceramics are extremely difficult and energy-intensive to melt and cast.
Sintering provides a more practical and economical method to form dense, strong parts from these high-performance materials at significantly lower temperatures.
Tailoring Material Microstructure
The controlled time and temperature of sintering allow for precise control over the final microstructure of the material.
This enables the engineering of specific properties like hardness, strength, and even controlled porosity, which are difficult to achieve through simple melting and solidification.
Understanding the Pitfalls and Trade-offs
The temperature window for successful sintering is often narrow, and deviation carries significant consequences.
The Risk of Undershooting the Temperature
If the sintering temperature is too low, atomic diffusion will be insufficient. The bonds between particles will be weak, leaving a porous and mechanically fragile part.
This results in a component that fails to meet density and strength specifications.
The Risk of Overshooting the Temperature
If the temperature is too high and approaches the melting point, it can lead to rapid and uncontrolled grain growth, which can make the material brittle.
Worse, any localized or partial melting can cause the part to distort, shrink unevenly, or lose its intended shape entirely.
The Energy and Control Equation
While sintering requires precise control, it is generally more energy-efficient than fully melting and casting an equivalent volume of material.
This trade-off—exchanging the brute force of high heat for the precision of controlled thermal energy—is a primary driver for its use in mass production.
Making the Right Choice for Your Goal
Your choice between leveraging sintering or melting depends entirely on your end goal for the material.
- If your primary focus is creating complex, net-shape parts from powders: Sintering is your ideal process, as it works below the melting point to preserve form and control properties.
- If your primary focus is creating a homogenous liquid for casting into a simple mold: Melting is the necessary first step, as you require a complete phase change from solid to liquid.
- If your primary focus is manufacturing parts from extremely high-melting-point materials: Sintering provides the only economically and technically viable path forward.
Mastering the relationship between sintering temperature and melting point is the key to unlocking the full potential of modern materials processing.

Summary Table:
| Key Aspect | Sintering | Melting |
|---|---|---|
| Temperature | Below Melting Point (50-90%) | Above Melting Point |
| Primary Goal | Densify & Strengthen Powder Compacts | Achieve Complete Liquefaction |
| Material State | Solid-State Diffusion | Liquid Phase |
| Shape Outcome | Preserves Complex Net Shape | Requires Casting/Molding |
| Ideal For | Powder Metallurgy, High-MP Materials (e.g., Tungsten) | Casting, Alloying |
Need precise thermal processing equipment to master sintering? KINTEK specializes in high-performance lab furnaces and consumables designed for controlled sintering applications. Our solutions help you achieve optimal density and strength while avoiding the pitfalls of incorrect temperatures. Contact our experts today to discuss your specific material and application requirements!
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- How do magnets enhance the sputtering rate in magnetron sputtering process and improve the thin film quality? Boost Deposition Speed & Film Quality
- How does press forging differ from drop forging? Control vs. Speed in Metal Forming
- How does a laboratory constant temperature drying oven ensure repeatability? Standardize Your Catalyst Cycling Results
- What is the purity of distillate? Achieving 90-99% Cannabinoid Potency
- What metals can you braze together? A Guide to Strong, Versatile Metal Joining
- What is the maintenance of laboratory equipment? Ensure Data Integrity and Extend Equipment Lifespan
- What is the density of synthetic graphite? Understanding the Range from 1.5 to 2.26 g/cm³
- What is the principle of quenching effect? Harnessing Molecular Interactions to Control Fluorescence



















