At its core, a sintering cycle is a precise, multi-stage thermal process designed to transform a loosely packed powder compact into a strong, dense, solid object. This is achieved by applying heat below the material's melting point, causing the individual particles to fuse together at their contact points, systematically reducing porosity and increasing the component's density and strength.
The fundamental purpose of a sintering cycle isn't just to heat a material, but to guide it through a carefully engineered temperature profile. This controlled journey removes temporary binders, promotes atomic diffusion between particles, and solidifies the part while achieving specific, desired material properties.

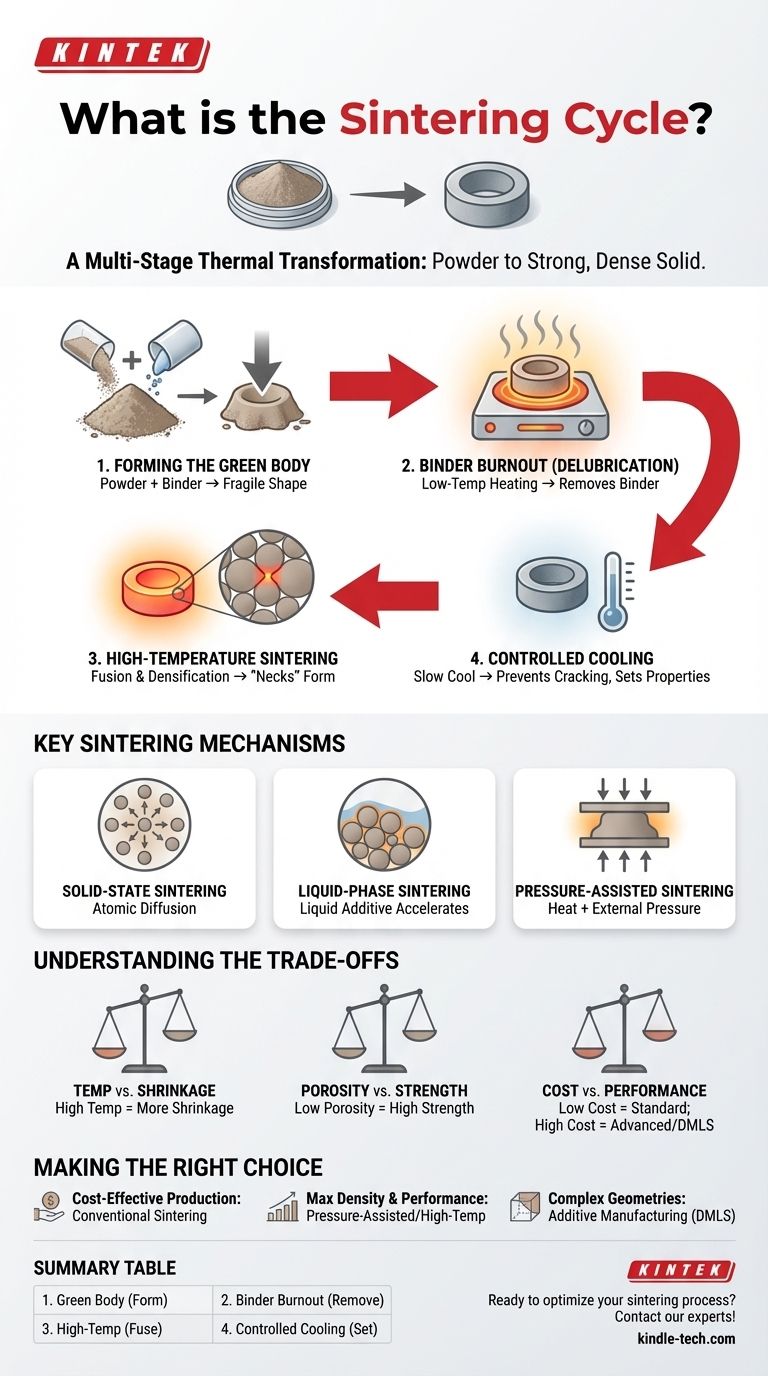
The Anatomy of a Sintering Cycle
The sintering cycle is best understood as a sequence of distinct thermal stages, each with a critical function. The rate of heating, holding times, and cooling are all meticulously controlled variables.
Stage 1: Forming the "Green Body"
Before any heating begins, the primary material powder is mixed with a temporary binder, such as wax, water, or a polymer. This mixture is then pressed into a desired shape, creating what is known as a "green body."
This green body is fragile and has low strength, as the particles are only held together by the binder.
Stage 2: Binder Burnout (Delubrication)
The first heating stage involves a slow ramp-up to a relatively low temperature. The primary goal is to completely and carefully burn off or evaporate the binder.
This step must be done slowly to allow the binder by-products to escape without building up pressure and causing cracks or defects in the part. In some processes, agents like water vapor are used to help convert these by-products into harmless gasses like CO2.
Stage 3: High-Temperature Sintering
Once the binder is removed, the temperature is raised significantly, approaching (but not reaching) the melting point of the primary material. This is where the actual sintering and densification occur.
At this high temperature, atoms gain enough energy to diffuse across the boundaries of adjacent particles. This atomic transport causes "necks" to form and grow at particle contact points, pulling the particles closer, eliminating the empty pores between them, and fusing the structure into a solid mass.
Stage 4: Controlled Cooling
After holding the part at the sintering temperature for a specified time, the final stage is a controlled cooling process.
The cooling rate is critical for preventing thermal shock and cracking. It also plays a crucial role in determining the final microstructure and, therefore, the mechanical properties of the finished component, such as its hardness and toughness.
Key Sintering Mechanisms
The high-temperature fusion stage can be achieved through different physical mechanisms, which define the type of sintering process used.
Solid-State Sintering
This is the most fundamental form of sintering. The component is made of a single powder, and fusion occurs entirely through atomic diffusion between the solid particles. It is a cost-effective and widely used method.
Liquid-Phase Sintering (LPS)
In this technique, a small amount of an additive with a lower melting point is mixed with the primary powder. During the high-temperature stage, this additive melts, creating a liquid phase that wets the solid particles.
The liquid accelerates densification through capillary action, which pulls particles together and allows for faster material transport as solid particles dissolve and re-precipitate to fill pores more efficiently.
Pressure-Assisted Sintering
Techniques like hot pressing apply external pressure simultaneously with high temperature. This mechanical force physically aids in closing pores and accelerating densification.
This method can achieve extremely high densities that are difficult to obtain through pressureless sintering alone, resulting in superior mechanical properties.
Understanding the Trade-offs
Choosing and designing a sintering cycle involves balancing competing factors. There is no single "best" cycle; there is only the best cycle for a specific application and material.
Temperature vs. Shrinkage
Higher sintering temperatures generally lead to faster diffusion, better densification, and improved mechanical properties. However, they also cause greater shrinkage of the component, which must be precisely accounted for in the initial mold design.
Porosity vs. Strength
The primary goal of most sintering is to eliminate porosity. Lower porosity almost always correlates with higher density, strength, and durability. However, for some applications like self-lubricating bearings or filters, a certain level of controlled, interconnected porosity is a desired design feature.
Cost vs. Performance
Conventional, pressureless sintering is a highly cost-effective method suitable for mass-producing parts like gears, pulleys, and sprockets. Advanced methods like hot pressing or Direct Metal Laser Sintering (DMLS) offer superior performance and geometric complexity but come at a significantly higher cost per part.
Making the Right Choice for Your Goal
Your choice of sintering method and cycle parameters depends entirely on the intended outcome for your component.
- If your primary focus is cost-effective mass production: Conventional solid-state or liquid-phase sintering of pressed green bodies is the industry standard for reliable, high-volume manufacturing.
- If your primary focus is achieving maximum density and mechanical performance: Pressure-assisted methods or higher-temperature cycles are necessary, accepting the trade-offs of higher cost and more complex process control.
- If your primary focus is creating complex geometries with high precision: Additive manufacturing techniques like DMLS apply sintering principles on a layer-by-layer basis, offering unparalleled design freedom.
Mastering the sintering cycle is key to transforming simple powders into high-performance engineered components.
Summary Table:
| Sintering Cycle Stage | Key Function |
|---|---|
| 1. Green Body Formation | Powder is mixed with a binder and pressed into the desired shape. |
| 2. Binder Burnout | Low-temperature heating to carefully remove the temporary binder. |
| 3. High-Temp Sintering | Heat near the melting point fuses particles, increasing density. |
| 4. Controlled Cooling | Slow cooling prevents cracking and sets final material properties. |
Ready to optimize your sintering process for superior part performance? KINTEK specializes in providing the high-performance lab equipment and consumables necessary for precise thermal processing. Whether you're engaged in R&D or high-volume production, our solutions help you achieve the perfect density, strength, and microstructure for your components. Contact our experts today to discuss your specific sintering needs!
Visual Guide
Related Products
- Spark Plasma Sintering Furnace SPS Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
People Also Ask
- Can aluminum be sintered? Overcome the Oxide Barrier for Complex, Lightweight Parts
- What is the vapor phase material? Unlock Faster, Denser Sintering with SPS Technology
- What is the plasma sintering technique? Achieve Rapid, High-Density Material Fabrication
- What are the different sintering methods? Choose the Right Technique for Your Material & Application
- What is the SPS process of spark plasma sintering? A Guide to Rapid, Low-Temperature Densification



















