At its core, the synthesis of carbon nanotubes (CNTs) via Chemical Vapor Deposition (CVD) is a controlled process where a carbon-containing gas is thermally decomposed over a metallic catalyst. The catalyst acts as a "seed," breaking down the gas and assembling the resulting carbon atoms into a cylindrical, tube-like structure. This method has become the dominant commercial process due to its scalability and superior control over the final product compared to older techniques like arc discharge or laser ablation.
Chemical Vapor Deposition is not merely a coating technique; for CNTs, it is a bottom-up catalytic growth process. The entire mechanism hinges on using a catalyst to controllably break down a carbon source and then reassemble the carbon atoms, atom by atom, into a highly ordered nanotube.

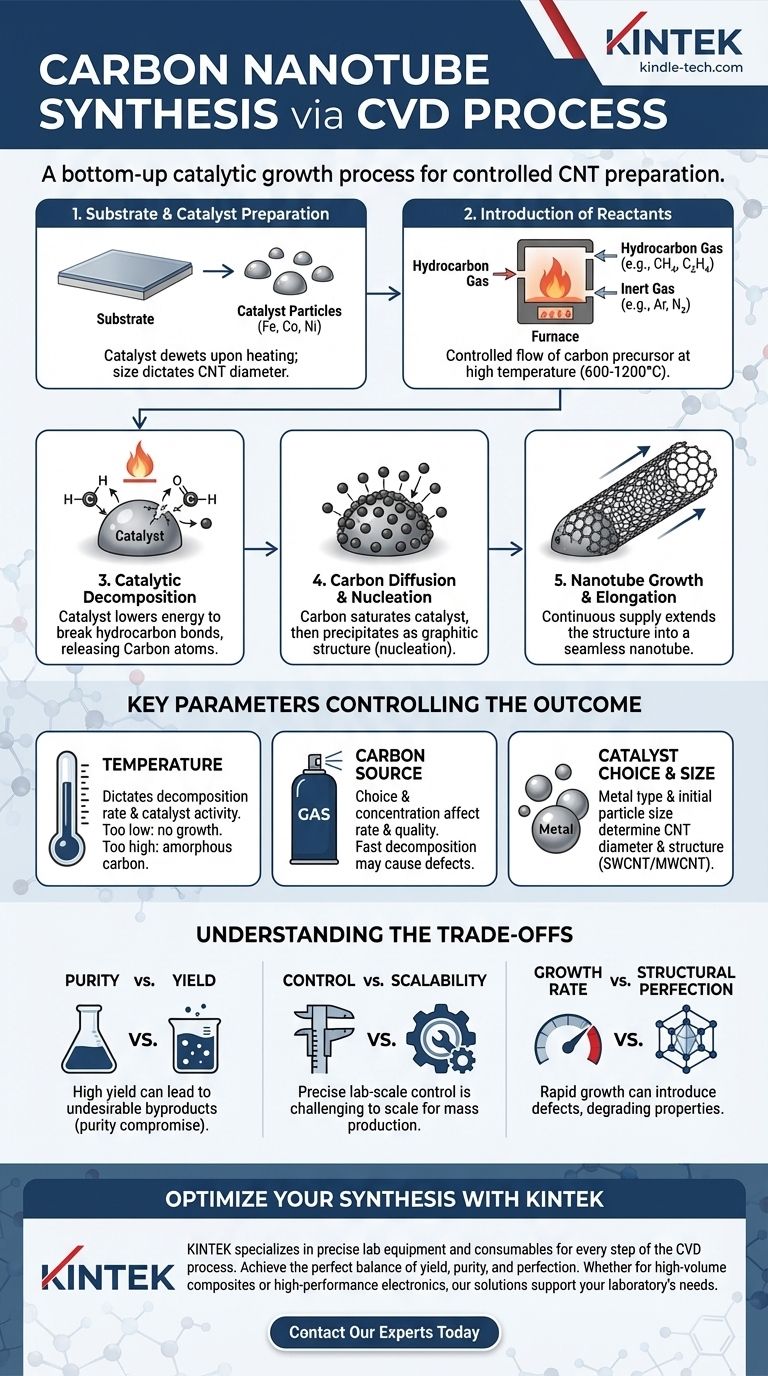
The Core Mechanism: A Step-by-Step Breakdown
To truly understand CVD for CNT synthesis, we must view it as a sequence of distinct physical and chemical events occurring at the nanoscale. Each step directly influences the quality and characteristics of the resulting nanotubes.
Step 1: Substrate and Catalyst Preparation
Before any reaction begins, a substrate is prepared with a thin layer of catalyst material. This is typically a transition metal like iron (Fe), cobalt (Co), or nickel (Ni).
Upon heating, this thin film breaks up into discrete nanoparticles due to a process called solid-state dewetting. The size of these nanoparticles is critical, as it often dictates the diameter of the nanotubes that will grow from them.
Step 2: Introduction of Reactants
The prepared substrate is placed inside a high-temperature furnace (typically 600-1200°C). A carefully controlled flow of gases is introduced.
This includes an inert carrier gas (like argon or nitrogen) and a carbon-containing precursor gas (a hydrocarbon such as methane, ethylene, or acetylene).
Step 3: Catalytic Decomposition
At the high process temperature, the hydrocarbon gas molecules do not simply deposit on the substrate. Instead, they are catalytically decomposed on the surface of the hot metal nanoparticles.
The catalyst's function is to significantly lower the energy required to break the chemical bonds in the hydrocarbon, releasing elemental carbon atoms.
Step 4: Carbon Diffusion and Nucleation
The liberated carbon atoms dissolve into or diffuse across the surface of the metal catalyst nanoparticle. The particle essentially becomes saturated with carbon.
Once the catalyst particle reaches its carbon solubility limit, the carbon begins to precipitate out from the particle in a stable, graphitic form. This precipitation marks the nucleation—the birth—of the nanotube wall.
Step 5: Nanotube Growth and Elongation
As more hydrocarbon decomposes, a continuous supply of carbon feeds the catalyst, causing the precipitated carbon structure to extend outwards, forming a seamless cylindrical tube.
This growth continues as long as the catalyst particle remains active and a supply of carbon precursor is available.
Key Parameters Controlling the Outcome
The final properties of the CNTs—such as their diameter, length, and purity—are not accidental. They are the direct result of precise control over several critical operating parameters.
Temperature
Temperature is arguably the most important variable. It dictates the decomposition rate of the carbon source and the activity of the catalyst. Too low, and no growth occurs; too high, and you may get amorphous carbon or other unwanted structures.
Carbon Source and Concentration
The choice of hydrocarbon gas and its concentration affects the growth rate and quality. Gases that decompose easily (like acetylene) can lead to faster growth but may also produce more defects and impurities.
Catalyst Choice and Size
The type of metal catalyst and the size of the initial nanoparticles are fundamental. They directly influence the diameter and even the structure (e.g., single-walled vs. multi-walled) of the resulting CNTs.
Understanding the Trade-offs
While CVD is a powerful technique, it is governed by a series of compromises. Understanding these trade-offs is crucial for any practical application.
Purity vs. Yield
Conditions that favor a high yield (i.e., growing a large quantity of material) often lead to the co-production of undesirable byproducts, such as amorphous carbon or other nanoparticles. This necessitates complex and often harsh post-processing purification steps.
Control vs. Scalability
Achieving precise control over nanotube diameter, length, and electronic properties (chirality) requires stringent, lab-scale conditions. Scaling these precise conditions up for industrial production is a significant engineering challenge, often forcing a compromise on the uniformity of the final product.
Growth Rate vs. Structural Perfection
Rapid growth rates can introduce defects into the carbon lattice of the nanotube walls. These imperfections can degrade the exceptional mechanical and electrical properties that make CNTs so valuable.
Making the Right Choice for Your Goal
Your approach to CVD synthesis should be dictated entirely by your end-goal. The optimal process for one application may be unsuitable for another.
- If your primary focus is high-volume production for composites: Prioritize high-yield conditions and a robust catalyst, as you can tolerate a wider distribution of nanotube diameters and lengths.
- If your primary focus is high-performance electronics: You must use stringent process control, highly pure precursors, and carefully engineered catalysts to produce nanotubes with minimal defects and the desired electronic properties.
- If your primary focus is fundamental research: Your goal is to isolate variables, using ultra-pure materials and precise control systems to systematically study how each parameter influences the growth mechanism itself.
Ultimately, mastering CVD for carbon nanotube synthesis is an exercise in the controlled manipulation of chemistry and physics at the atomic scale.
Summary Table:
| CVD Step | Key Action | Critical Parameter |
|---|---|---|
| Step 1: Preparation | Substrate coated with catalyst (Fe, Co, Ni) | Catalyst particle size |
| Step 2: Reactant Intro | Hydrocarbon gas (e.g., methane) flows into furnace | Gas concentration & flow rate |
| Step 3: Decomposition | Catalyst breaks down carbon source at high temp (600-1200°C) | Temperature & catalyst activity |
| Step 4: Nucleation | Carbon precipitates from saturated catalyst | Carbon solubility limit |
| Step 5: Growth | Continuous carbon feed elongates the nanotube | Growth duration & carbon supply |
Ready to optimize your carbon nanotube synthesis? KINTEK specializes in providing the precise lab equipment and consumables—from CVD furnaces to high-purity catalysts and gases—needed to control every step of the CNT growth process. Whether you're scaling up for composites or refining for electronics, our solutions help you achieve the right balance of yield, purity, and structural perfection. Contact our experts today to discuss how we can support your laboratory's specific needs!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What are the advantages of industrial CVD for solid boriding? Superior Process Control and Material Integrity
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision



















