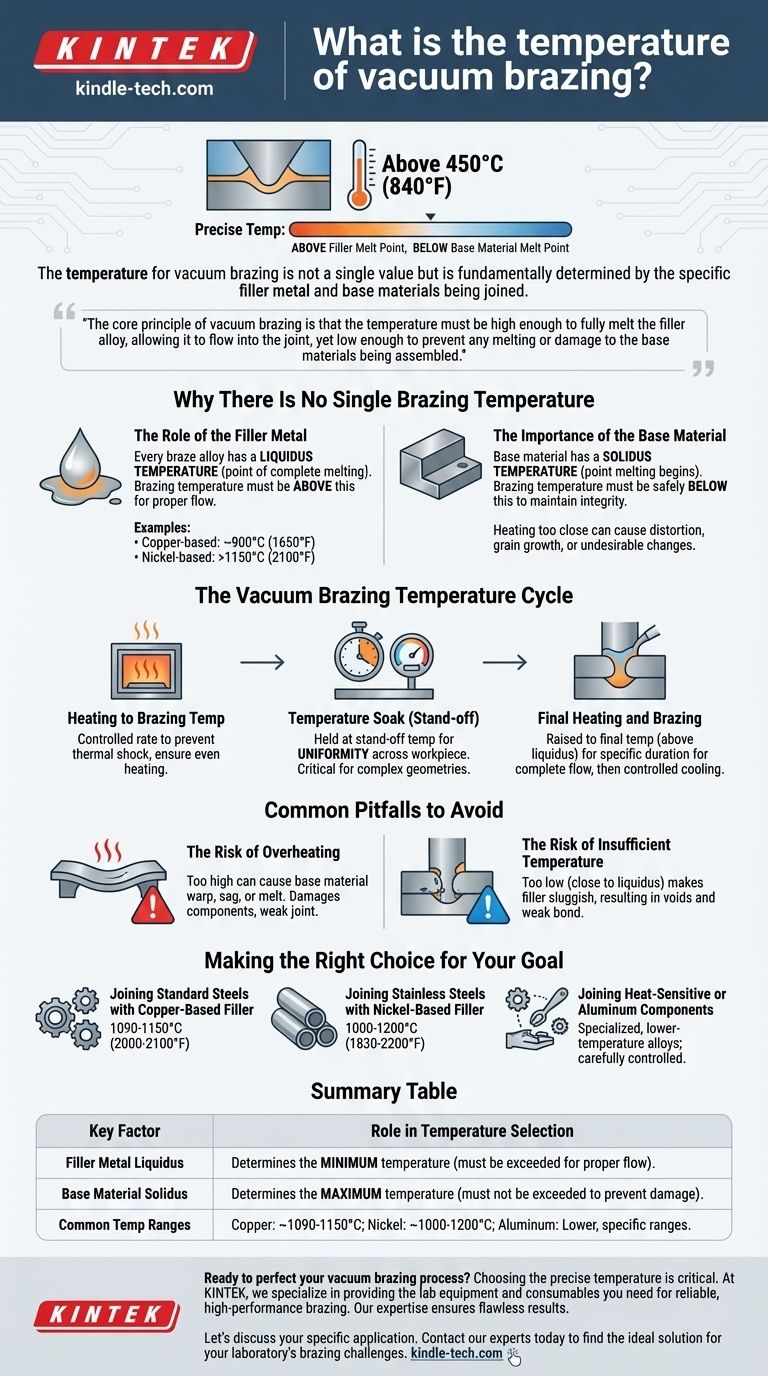
The temperature for vacuum brazing is not a single value but is fundamentally determined by the specific filler metal and base materials being joined. While all brazing occurs above 450°C (840°F), the precise temperature is carefully chosen to be above the filler metal's melting point but safely below the melting point of the components being bonded.
The core principle of vacuum brazing is that the temperature must be high enough to fully melt the filler alloy, allowing it to flow into the joint, yet low enough to prevent any melting or damage to the base materials being assembled.

Why There Is No Single Brazing Temperature
The selection of a brazing temperature is a critical engineering decision based on the metallurgy of the assembly. It is a balancing act between the properties of the filler metal and the base materials.
The Role of the Filler Metal
The primary factor dictating the minimum brazing temperature is the filler metal, also known as the braze alloy.
Every braze alloy has a liquidus temperature, which is the point at which it becomes completely liquid. The brazing temperature must be set above this liquidus point to ensure the alloy can flow freely into the joint via capillary action.
For example, some copper-based alloys may become fully liquid around 900°C (1650°F), while high-performance nickel-based alloys might require temperatures exceeding 1150°C (2100°F).
The Importance of the Base Material
The second critical factor is the base material of the parts being joined.
The base material has a solidus temperature, the point at which it begins to melt. The brazing temperature must always remain safely below this point to maintain the structural integrity of the workpiece.
Heating a base metal too close to its solidus can cause distortion, grain growth, or other undesirable metallurgical changes, even if it doesn't melt.
The Vacuum Brazing Temperature Cycle
The final brazing temperature is just one point in a carefully controlled heating and cooling cycle. Each stage serves a distinct purpose.
Heating to Brazing Temperature
The furnace heats the components at a controlled rate. This slow, steady increase prevents thermal shock and allows all parts of the assembly, thick and thin, to heat up evenly.
Temperature Soak (Stand-off)
Before reaching the final brazing temperature, the cycle often includes a "soak." The furnace holds the assembly at a stand-off temperature for a period to ensure complete temperature uniformity across the entire workpiece.
This step is critical for complex geometries, ensuring that when the filler metal melts, all parts of the joint are at the correct temperature to accept it.
Final Heating and Brazing
After the soak, the temperature is raised to the final brazing point, above the filler's liquidus. It is held here for a specific duration to allow the filler to flow completely throughout the joint, after which a controlled cooling cycle begins.
Common Pitfalls to Avoid
Setting the wrong temperature can lead to complete failure of the brazed joint. Understanding the risks is key to a successful process.
The Risk of Overheating
Setting the temperature too high can be catastrophic. It can cause the base material to warp, sag, or even begin to melt. This damages the components and creates a weak, unreliable joint.
The Risk of Insufficient Temperature
Setting the temperature too low, or too close to the filler's liquidus, is also a common failure mode. The filler metal will be sluggish and will not flow properly, resulting in voids, incomplete joint fill, and a weak bond.
Making the Right Choice for Your Goal
The correct temperature is always derived from the material specifications provided by the filler metal manufacturer and a thorough understanding of your base materials.
- If your primary focus is joining standard steels with a copper-based filler: Your brazing temperature will likely be in the range of 1090-1150°C (2000-2100°F).
- If your primary focus is joining stainless steels with a nickel-based filler: You will be operating at higher temperatures, often between 1000-1200°C (1830-2200°F), depending on the specific alloy.
- If your primary focus is joining heat-sensitive or aluminum components: You will use specialized, lower-temperature filler alloys, with brazing temperatures carefully controlled to avoid damaging the base metal.
Ultimately, successful vacuum brazing depends on selecting a temperature that creates perfect fluidity for the filler without compromising the integrity of the workpiece.
Summary Table:
| Key Factor | Role in Temperature Selection |
|---|---|
| Filler Metal Liquidus | Determines the minimum temperature (must be exceeded for proper flow). |
| Base Material Solidus | Determines the maximum temperature (must not be exceeded to prevent damage). |
| Common Temperature Ranges | Copper alloys: ~1090-1150°C; Nickel alloys: ~1000-1200°C; Aluminum alloys: Lower, specific ranges. |
Ready to perfect your vacuum brazing process?
Choosing the precise temperature is critical for joint strength and component integrity. At KINTEK, we specialize in providing the lab equipment and consumables you need for reliable, high-performance brazing. Our expertise ensures you can achieve flawless results, whether you're working with standard steels, stainless steels, or heat-sensitive materials.
Let's discuss your specific application. Contact our experts today to find the ideal solution for your laboratory's brazing challenges.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What role does a laboratory oven play during the curing phase of NIPU coatings? Ensure Superior Cross-Linking
- Why is a Vacuum Drying Oven used for anhydrous Na3B24H23? Ensure Purity for Solid Electrolytes
- What is the carburizing process in heat treatment? Create Wear-Resistant Parts with a Tough Core
- How is an industrial electric furnace utilized to evaluate HVOF coatings? Optimize Thermal Fatigue Performance
- What precautions should be taken during heat treatment? Essential Safety and Quality Control Measures
- What kind of energy does pyrolysis generate? Converting Waste into Valuable Fuels
- What is the significance of using high-vacuum heat treatment furnaces and rapid quenching for zirconium alloys?
- Why is a high-purity gas supply system necessary for ion carburizing? Ensure Precise Surface Integrity and Phase Purity



















