In modern science and medicine, potassium bromide (KBr) serves two primary, yet distinct, roles. It is an essential material in the field of infrared spectroscopy due to its unique optical properties, and it remains a specialized veterinary medicine for controlling epileptic seizures in dogs. Its historical use as a sedative and anticonvulsant in humans is now obsolete due to its toxicity.
Potassium bromide's story is one of transition. Once a widespread but hazardous human medicine, it has evolved into a niche but indispensable tool for specific applications in scientific analysis and veterinary care where its unique properties are unparalleled.

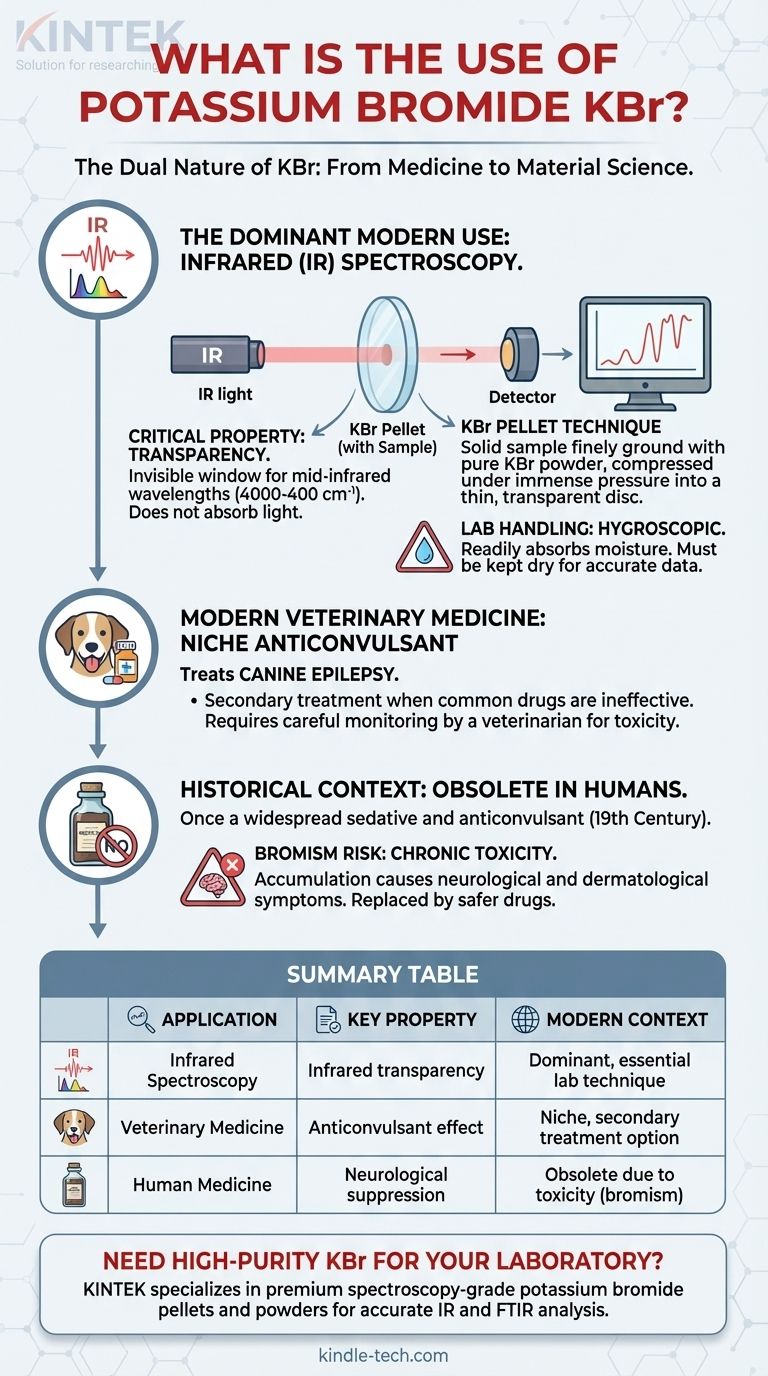
The Dual Nature of KBr: From Medicine to Material Science
Potassium bromide is an ionic salt with a history stretching back to the 19th century. Its journey reveals how a chemical's utility is defined not just by its effects, but also by our understanding of its risks and the development of superior alternatives.
Historical Medical Use: The First Effective Anti-Epileptic
In 1857, Sir Charles Locock discovered that potassium bromide could control seizures in patients with epilepsy, making it the first effective anticonvulsant. This discovery was a landmark in neurology.
Following this, it was widely prescribed as a sedative and hypnotic for a range of nervous disorders, from anxiety to insomnia. Its use was so common that the term "take a bromide" became an idiom for a calming platitude.
Modern Veterinary Medicine: A Niche Anticonvulsant Role
While no longer used in humans, KBr is still prescribed by veterinarians to treat canine epilepsy. It is often used as a secondary treatment when more common drugs like phenobarbital are ineffective or cause unacceptable side effects.
Its use requires careful monitoring by a veterinarian to manage dosage and watch for signs of toxicity, as dogs can also suffer from the side effects of bromide accumulation.
The Dominant Modern Use: Infrared (IR) Spectroscopy
Today, the most significant and widespread application of potassium bromide is in the field of analytical chemistry, specifically infrared (IR) spectroscopy. This technique is used to identify chemical compounds by analyzing how they absorb infrared light.
Why KBr? The Critical Property of Transparency
The primary reason KBr is used is its transparency to infrared radiation. Across a very wide range of mid-infrared wavelengths (approximately 4000 to 400 cm⁻¹), a pure KBr crystal does not absorb the light itself.
Think of it as a perfectly clean, invisible window for the infrared spectrum. This ensures that any absorption measured by the spectrometer comes from the sample being analyzed, not from the KBr holding it.
The KBr Pellet Technique
For analyzing solid samples, the KBr pellet method is a standard procedure. A small amount of the solid sample is finely ground with pure, dry KBr powder.
This mixture is then compressed under immense pressure (tons per square inch) in a die. The pressure fuses the KBr powder into a thin, transparent disc, or "pellet," with the sample material suspended evenly throughout. The IR beam can then pass through this pellet for analysis.
Other Optical Applications
Beyond pellets, high-purity KBr is also used to manufacture optical components like windows, lenses, and prisms for IR and Fourier-transform infrared (FTIR) spectrometers and other optical devices designed to work in the infrared spectrum.
Understanding the Trade-offs and Safety Concerns
The decline of KBr in human medicine and the careful handling required in the lab are both rooted in its chemical properties and biological effects.
Bromism: The Risk of Chronic Toxicity
The primary reason KBr was abandoned for human use is its potential to cause bromism, a state of chronic bromide intoxication. Because bromide ions have a very long half-life in the body (around 12 days), they can accumulate with repeated dosing.
Symptoms of bromism are primarily neurological and dermatological, including lethargy, depression, memory loss, psychosis, ataxia (loss of coordination), and a specific skin rash known as bromide acne. The development of safer, more effective drugs like barbiturates and later benzodiazepines made KBr obsolete.
Handling in the Laboratory
In a laboratory setting, the main concern is KBr's hygroscopic nature—it readily absorbs moisture from the atmosphere. A "wet" KBr pellet or window will show strong water absorption bands in the IR spectrum, potentially obscuring the sample's true data.
For this reason, spectroscopy-grade KBr must be kept scrupulously dry, typically stored in a desiccator and often heated in an oven before use to drive off any absorbed water. Standard laboratory personal protective equipment, like gloves and safety glasses, should be worn when handling the powder.
Applying This Knowledge to Your Context
Your perspective on potassium bromide depends entirely on your field. It can be seen as a historical artifact, a dangerous substance, or an essential analytical tool.
- If your primary focus is chemical analysis: Recognize KBr as the gold standard material for preparing solid samples for IR spectroscopy due to its unparalleled infrared transparency, but remember its hygroscopic nature demands careful, dry handling.
- If your primary focus is veterinary medicine: View KBr as a valuable second-line anticonvulsant for dogs, effective but requiring strict veterinary supervision to manage the significant risk of toxicity.
- If your primary focus is medical history: See KBr as a foundational but now obsolete pharmaceutical, representing a critical first step in treating epilepsy that was rightfully superseded by far safer and more effective alternatives.
Understanding potassium bromide's journey from a common medicine to a specialized scientific tool highlights the constant evolution of chemical applications driven by our ever-improving knowledge of efficacy and safety.
Summary Table:
| Application | Primary Use | Key Property | Modern Context |
|---|---|---|---|
| Infrared Spectroscopy | Sample preparation for chemical analysis | Infrared transparency | Dominant, essential lab technique |
| Veterinary Medicine | Treatment for canine epilepsy | Anticonvulsant effect | Niche, secondary treatment option |
| Human Medicine | Historic sedative/anticonvulsant | Neurological suppression | Obsolete due to toxicity (bromism) |
Need High-Purity KBr for Your Laboratory?
KINTEK specializes in providing premium lab equipment and consumables, including spectroscopy-grade potassium bromide (KBr) pellets and powders. Our products ensure accurate, reliable results for your IR and FTIR analysis, helping you identify chemical compounds with precision.
We serve laboratories requiring dependable materials for analytical chemistry, research, and quality control.
Contact us today via our form to discuss your specific needs and discover how KINTEK can support your laboratory's success with our high-quality consumables and expert service.
Visual Guide
Related Products
- Electrode Polishing Material for Electrochemical Experiments
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
People Also Ask
- How does sample size affect analysis? Maximize the Reliability of Your Research
- What is the recommended polishing sequence for a disk electrode that has scratches? Restore Your Surface to a Mirror Finish
- What is the importance of electrolytic polishing and electrolytic cells in FeCrAl sample prep? Reveal true structures.
- What are the two methods that can be used to prevent corrosion of a metal? Barrier vs. Sacrificial Protection Explained
- How long does it take to solder? A guide to timing and technique for perfect joints



















