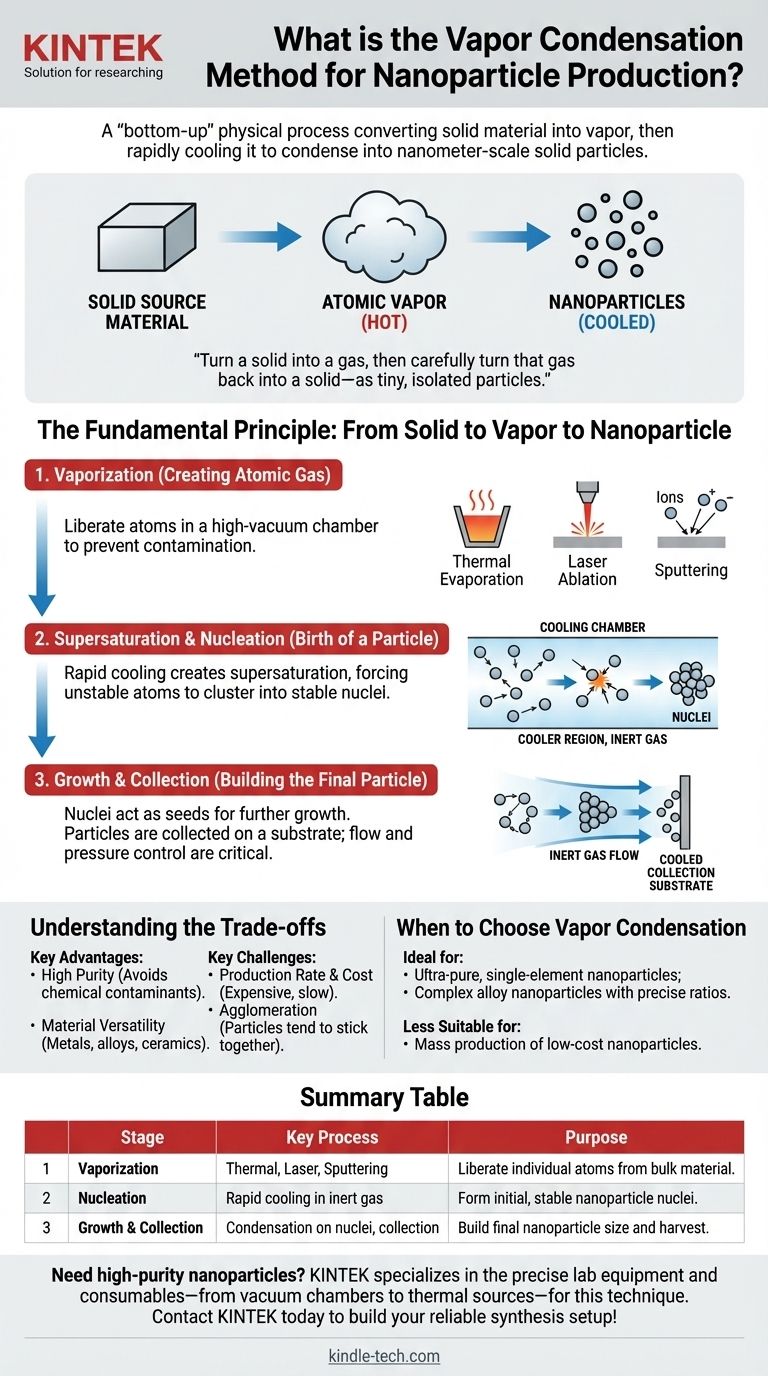
The vapor condensation method is a "bottom-up" physical process for producing nanoparticles. It works by first converting a solid material into a vapor, then causing the vaporized atoms to cool rapidly in a controlled environment. This cooling forces the atoms to cluster together and condense into solid particles on the nanometer scale.
The core principle is simple: turn a solid into a gas, then carefully turn that gas back into a solid—but in the form of tiny, isolated particles. Success hinges on precisely controlling the temperature and pressure to dictate when and how the atoms reassemble.

The Fundamental Principle: From Solid to Vapor to Nanoparticle
The entire process can be broken down into three critical physical stages. Each stage offers a point of control to fine-tune the final nanoparticle characteristics.
Step 1: Vaporization (Creating an Atomic Gas)
The first objective is to liberate individual atoms from the bulk source material, creating a hot atomic vapor. This is typically done inside a high-vacuum chamber to prevent contamination.
Common vaporization techniques include:
- Thermal Evaporation: The material is heated in a crucible until it boils and evaporates.
- Laser Ablation: A high-power laser pulse strikes the material, instantly vaporizing a small amount of the surface.
- Sputtering: As described in Physical Vapor Deposition (PVD), the source material (a "target") is bombarded with high-energy inert gas ions (like Argon), which physically knock atoms off the surface.
Step 2: Supersaturation and Nucleation (The Birth of a Particle)
This is the most crucial stage. The hot atomic vapor is directed into a cooler region, typically filled with a low-pressure inert gas (e.g., helium or argon).
The rapid cooling creates a supersaturated state. This means the local concentration of vapor atoms is much higher than what the cool gas can normally hold, making the system unstable. To regain stability, atoms begin colliding and sticking together, forming the initial, stable clusters known as nuclei. This is the birth of the nanoparticles.
Step 3: Growth and Collection (Building the Final Particle)
Once nuclei have formed, they act as seeds for further growth. More atoms from the vapor phase condense onto these nuclei, causing the particles to grow in size.
The nanoparticles are then carried by the gentle flow of the inert gas to a collection surface. This is often a cooled substrate or filter where they can be harvested. Controlling the gas pressure and flow rate is critical here to prevent particles from growing too large or clumping together excessively.
Understanding the Trade-offs
Like any fabrication technique, the vapor condensation method has distinct advantages and challenges that make it suitable for specific applications.
Key Advantage: High Purity
Because the process occurs in a highly controlled vacuum or inert gas environment, the resulting nanoparticles can be exceptionally pure. This method avoids the chemical precursors and solvents used in wet chemical synthesis, eliminating a major source of contamination.
Key Advantage: Material Versatility
This physical method is effective for a wide range of materials that can be vaporized. It is especially well-suited for producing nanoparticles from pure metals, alloys, and certain ceramic oxides.
Key Challenge: Production Rate and Cost
Vapor condensation typically requires sophisticated high-vacuum equipment, which is expensive to acquire and operate. The process can be slow and energy-intensive, making it less economical for large-scale, bulk production compared to chemical methods.
Key Challenge: Agglomeration
Nanoparticles have extremely high surface energy, giving them a powerful natural tendency to stick together, or agglomerate. Preventing this during the collection and handling phases is a significant engineering challenge that can affect the final product's usability.
When to Choose the Vapor Condensation Method
Deciding if this method is right for your goal depends entirely on the required purity, material type, and production scale.
- If your primary focus is creating ultra-pure, single-element nanoparticles for research or high-performance electronics: Vapor condensation is an excellent choice due to its clean, contaminant-free process.
- If your primary focus is fabricating complex alloy nanoparticles with precise elemental ratios: This method offers superior control, as multiple source materials can be vaporized simultaneously to create homogenous nano-alloys.
- If your primary focus is mass production of low-cost nanoparticles (e.g., for pigments or bulk composites): This method is likely unsuitable due to its lower production rates and higher operational costs.
Mastering this technique comes from understanding that you are fundamentally controlling the transition of matter from a solid to a gas and back again.
Summary Table:
| Stage | Key Process | Purpose |
|---|---|---|
| 1. Vaporization | Thermal Evaporation, Laser Ablation, Sputtering | Liberate individual atoms from bulk material. |
| 2. Nucleation | Rapid cooling in inert gas | Form initial, stable nanoparticle nuclei. |
| 3. Growth & Collection | Condensation on nuclei, collection on substrate | Build final nanoparticle size and harvest particles. |
Need high-purity nanoparticles for your research or advanced materials? The vapor condensation method is ideal for applications where material purity is paramount. KINTEK specializes in providing the precise lab equipment and consumables—from vacuum chambers to thermal sources—required to master this technique. Let our experts help you build a reliable nanoparticle synthesis setup. Contact KINTEK today to discuss your specific laboratory needs!
Visual Guide
Related Products
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- How is a high-temperature tube furnace utilized in the synthesis of SPAN? Optimize Your Li-S Battery Research Today
- Why is a controlled atmosphere tube furnace required for HPS catalysts? Ensure Optimal Metal Site Activation
- How does a three-zone high-temperature split tube furnace ensure data accuracy in creep experiments? Achieve Thermal Precision
- What are the advantages of using multi-stage split tube furnaces for heating methane pyrolysis reactors? Boost Efficiency
- What function does a high-temperature tube furnace serve in alkali fusion hydroxide recovery? Precision Thermal Control



















