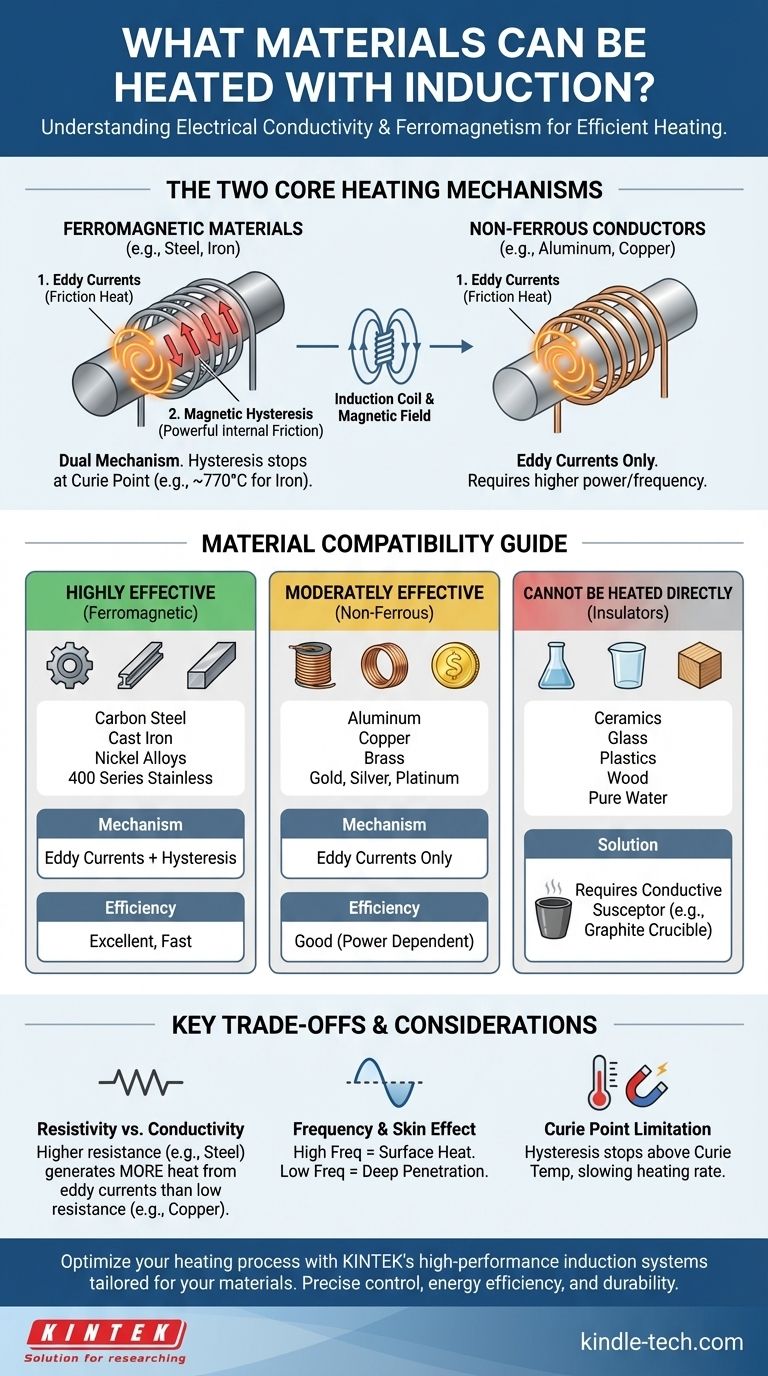
Fundamentally, induction heating works on electrically conductive materials, with its efficiency varying based on magnetic properties. This means that while metals like copper, aluminum, gold, and silver can be heated, ferrous metals such as iron and steel respond dramatically better to the process.
The ability to heat a material with induction is determined by two physical properties: electrical conductivity, which enables heating through eddy currents, and ferromagnetism, which adds a powerful secondary heating effect through hysteresis. While nearly any metal can be heated, ferrous metals are the most efficient because they benefit from both.

The Core Principles of Induction Heating
To understand which materials work best, you must first understand the two phenomena that generate heat in an induction system. They often work together but have different requirements.
The Role of Eddy Currents
An induction heater creates a powerful, alternating magnetic field. When an electrically conductive material is placed inside this field, the field induces small, circular electrical currents within the material. These are called eddy currents.
Every material has some resistance to the flow of electricity. As these eddy currents swirl through the material against its natural electrical resistance, they generate friction and precise, rapid heat. This is the primary way non-magnetic metals like aluminum, copper, and brass are heated.
The Power of Magnetic Hysteresis
The second, and often more powerful, heating effect only occurs in ferromagnetic materials. These include iron, nickel, cobalt, and most types of steel.
The magnetic particles within these materials resist the rapid back-and-forth switching of the induction coil's magnetic field. This internal friction generates significant heat. This effect, called hysteresis loss, is extremely efficient but only works below a specific temperature known as the Curie point.
Why Ferrous Metals Heat Best
Ferrous metals are ideal for induction because they benefit from both heating mechanisms simultaneously. They have the eddy currents common to all conductors, plus the intense internal friction from hysteresis.
Once the metal reaches its Curie temperature (around 770°C / 1420°F for iron), it loses its magnetic properties, and the hysteresis effect stops. From that point on, heating continues solely through the less-efficient eddy current effect.
A Practical Guide to Inductible Materials
Materials can be grouped into three simple categories based on their response to induction.
Highly Effective Materials (Ferromagnetic)
These materials heat quickly and efficiently due to the combined effect of eddy currents and hysteresis.
- Carbon Steels: Excellent candidates for induction due to high magnetic permeability and electrical resistance.
- Cast Iron: Responds very well, similar to carbon steel.
- Nickel and Cobalt Alloys: These magnetic metals also heat exceptionally well.
- Certain Stainless Steels: Ferritic and martensitic grades (like the 400 series) are magnetic and work well. Austenitic grades (like 304 or 316) are non-magnetic and behave like non-ferrous metals.
Moderately Effective Materials (Non-Ferrous Conductors)
These materials can only be heated by eddy currents and generally require higher frequencies or more power to reach the target temperature.
- Aluminum
- Copper
- Brass
- Gold, Silver, and Platinum
Materials That Cannot Be Heated
Materials that are electrical insulators cannot be heated directly by induction because there is no path for eddy currents to flow.
- Ceramics
- Glass
- Plastics
- Wood
- Water (unless it contains conductive ions)
To heat these materials, a conductive susceptor, such as a graphite crucible, is heated by induction, and the heat is then transferred to the non-conductive material via conduction or radiation.
Understanding the Key Trade-offs
Simply knowing a material is "inductive" is not enough. The efficiency of the process depends on several factors that create important trade-offs.
Resistivity vs. Conductivity
It may seem counterintuitive, but a material having lower electrical conductivity (higher resistivity) often heats better with eddy currents. While copper is an excellent conductor, its low resistance allows eddy currents to flow with little friction, generating less heat. Steel's higher resistance creates more heat from the same amount of current.
Frequency and the Skin Effect
The frequency of the alternating magnetic field determines how deep the heat penetrates. Higher frequencies keep the currents concentrated at the surface (the "skin effect"), which is ideal for surface hardening or heating small parts. Lower frequencies penetrate deeper, which is better for melting or through-heating large billets.
The Curie Point Limitation
Remember that the powerful hysteresis effect in ferrous metals disappears above the Curie point. This means the rate of heating will noticeably slow down once a piece of steel glows red-hot, as the work is then being done only by eddy currents.
Making the Right Choice for Your Goal
Your application dictates which material properties are most important.
- If your primary focus is rapid, high-efficiency heating (e.g., forging, hardening): Prioritize ferrous metals like carbon steel and iron to leverage the powerful dual-heating mechanism.
- If your primary focus is melting non-ferrous metals (e.g., aluminum, copper, precious metals): Induction is very effective, but you must ensure your system is designed with the appropriate power and frequency for eddy-current-only heating.
- If you are working with non-conductive materials (e.g., ceramics, glass): Direct induction heating is not an option; you must use a conductive susceptor like a graphite crucible to act as the heating element.
Understanding a material's electrical and magnetic properties is the key to mastering the power of induction heating.
Summary Table:
| Material Category | Key Examples | Heating Efficiency | Primary Mechanism |
|---|---|---|---|
| Highly Effective (Ferromagnetic) | Carbon Steel, Cast Iron, Nickel Alloys | Excellent | Eddy Currents + Magnetic Hysteresis |
| Moderately Effective (Non-Ferrous) | Aluminum, Copper, Brass, Gold, Silver | Good | Eddy Currents Only |
| Cannot Be Heated Directly | Ceramics, Plastics, Glass, Wood | Not Applicable | Requires Conductive Susceptor |
Ready to optimize your heating process with the right equipment? KINTEK specializes in high-performance lab furnaces and induction heating systems tailored for materials like steel, aluminum, and precious metals. Our solutions ensure precise temperature control, energy efficiency, and durability for your laboratory needs. Contact us today to discuss how we can enhance your lab's capabilities!
Visual Guide
Related Products
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Lab-Scale Vacuum Induction Melting Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the advantages of using a Vacuum Hot Press (VHP) furnace for sintering Zinc Sulfide? Achieve Optical Precision
- What are the advantages of using a vacuum hot pressing furnace over HIP? Optimize Fiber-Foil Composite Production
- What are the core advantages of using a vacuum hot pressing furnace for Cu/WC composites? Superior Density & Bonding
- What role does a vacuum hot pressing sintering furnace play in the fabrication of CuCrFeMnNi alloys? Achieve High Purity
- How does the vacuum hot pressing process improve wettability? Unlock Superior Diamond Product Bonding Strength


















