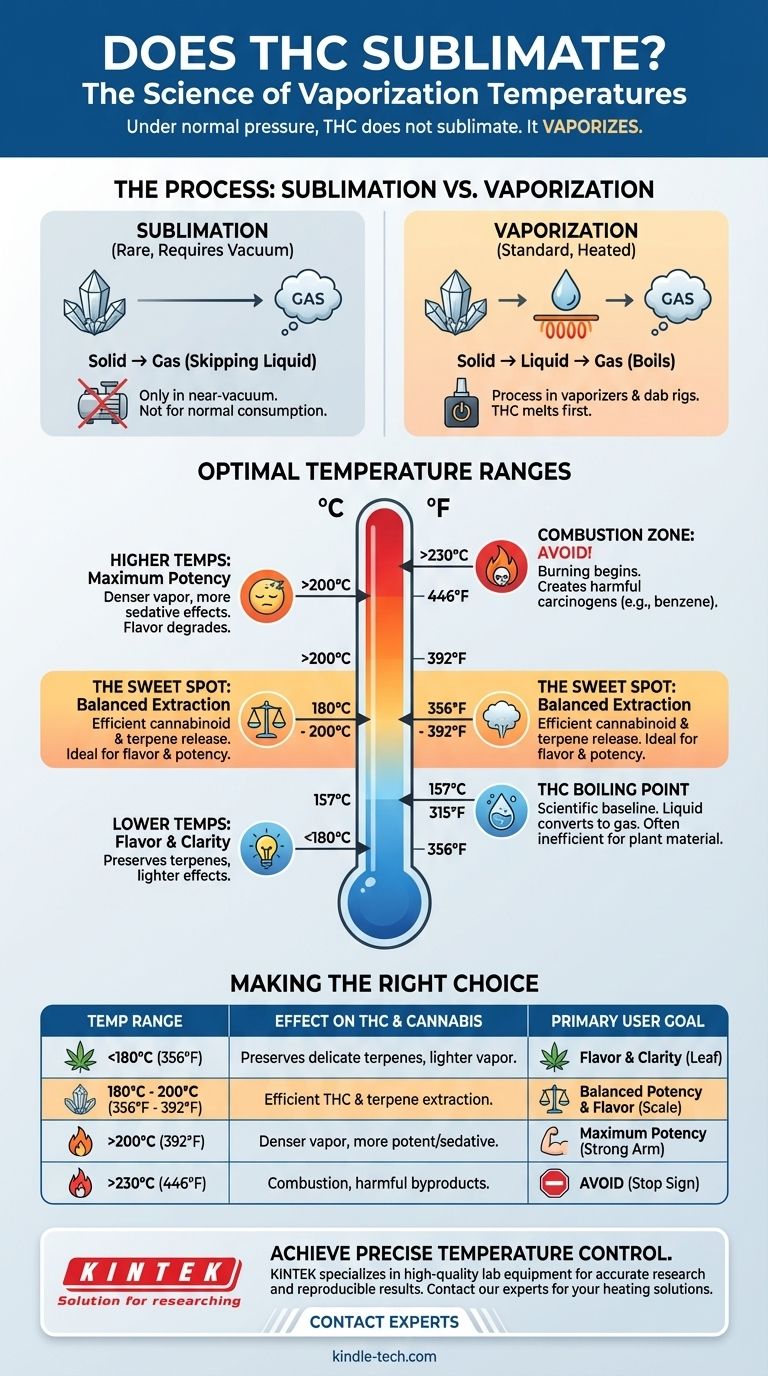
Under standard atmospheric pressure, THC does not sublimate. True sublimation—the direct transition from a solid to a gas—only occurs under a near-vacuum. The question you are likely asking relates to THC's boiling point, which is approximately 157°C (315°F), and its optimal vaporization range for consumption, which is considerably higher.
The critical distinction is not sublimation, but vaporization. While THC technically boils at 157°C (315°F), achieving the desired effects and flavor profile from cannabis requires a higher temperature range to efficiently release both cannabinoids and terpenes from the plant material without causing combustion.

Sublimation vs. Vaporization: What's Really Happening?
Understanding the physical process is key to controlling the outcome. The terms are often used incorrectly, leading to confusion about ideal temperatures.
The Technical Definition of Sublimation
Sublimation is a phase transition where a substance goes directly from a solid to a gas, completely skipping the liquid phase. The most common example of this is dry ice (solid carbon dioxide) turning into a cloud of gas at room temperature.
Why THC Doesn't Sublimate Under Normal Conditions
For a substance to sublimate, the surrounding atmospheric pressure must be below its "triple point." For THC, this requires a significant vacuum. In the context of any normal consumption method, from vaping to dabbing, this condition is never met. THC will always melt into a liquid before it becomes a gas.
Vaporization: The Process You Care About
Vaporization is the process of a substance in a liquid state turning into a gaseous state. This is what happens in a vaporizer or on a dab rig. The heat turns the solid cannabis resin into a liquid oil, which then boils and turns into the vapor you inhale. Effective vaporization begins below the boiling point and becomes highly efficient at and above it.
The Optimal Temperature Range for THC
Finding the right temperature is a balancing act. You need enough heat to activate the compounds you want, but not so much that you destroy them or burn the material.
The Boiling Point: A Scientific Baseline
The scientifically established boiling point of Delta-9-THC is 157°C (315°F). At this temperature, liquid THC actively converts into a gas. However, heating cannabis flower to only this temperature would be inefficient.
The "Sweet Spot" for Vaping
Most experienced users and studies agree that the ideal range for vaporizing cannabis is between 180°C to 200°C (356°F to 392°F). This range provides a comprehensive extraction of both cannabinoids and flavorful terpenes.
Why This Range Is Higher Than the Boiling Point
You are not heating pure, isolated THC. You are heating a complex plant material that contains dozens of compounds. This higher temperature range is necessary to efficiently release THC that is trapped within the plant's trichomes and to vaporize other beneficial compounds, like terpenes and other cannabinoids, which have their own unique boiling points.
Understanding the Trade-offs: Temperature and Its Effects
The temperature you choose directly dictates the flavor, potency, and harshness of the vapor. There is no single "best" temperature; it depends entirely on your goal.
Lower Temperatures (Below 180°C / 356°F)
At the lower end of the spectrum, you preserve the volatile terpenes, which are responsible for the cannabis's distinct aroma and flavor. The vapor is smoother and the psychoactive effects are often described as clearer and more "heady." The trade-off is a potentially less efficient THC extraction.
Higher Temperatures (Above 200°C / 392°F)
As you increase the temperature, you ensure the full vaporization of THC, leading to denser vapor and more potent, sedative effects. However, this comes at the cost of flavor, as the heat begins to degrade the delicate terpenes, which can result in a harsher taste.
The Danger Zone: Combustion
The combustion point of cannabis plant material is approximately 230°C (446°F). Once you exceed this temperature, you are no longer vaporizing; you are burning. This process creates smoke, which contains harmful carcinogens and toxins like benzene, defeating one of the primary health advantages of vaping.
Making the Right Choice for Your Goal
Use temperature to precisely tailor your experience. Your ideal setting will depend on what you want to prioritize.
- If your primary focus is flavor and a lighter effect: Start at a lower temperature, around 175-185°C (347-365°F), to preserve delicate terpenes.
- If your primary focus is maximizing THC potency and vapor density: Aim for a medium-high temperature, around 190-210°C (374-410°F), for a more intense effect.
- If your primary focus is avoiding harmful byproducts: Always stay below 230°C (446°F) to ensure you are vaporizing, not burning, the material.
Ultimately, understanding these temperature ranges gives you precise control over your experience.
Summary Table:
| Temperature Range | Effect on THC & Cannabis | Primary User Goal |
|---|---|---|
| Below 180°C (356°F) | Preserves terpenes, lighter effects | Flavor and clarity |
| 180°C - 200°C (356°F - 392°F) | Efficient THC and terpene extraction | Balanced potency and flavor |
| Above 200°C (392°F) | Denser vapor, more potent effects | Maximum potency |
| Above 230°C (446°F) | Combustion begins, harmful byproducts | AVOID (Burning) |
Achieve Precise Temperature Control in Your Lab
Understanding the exact vaporization point of compounds like THC is critical for research and product development. KINTEK specializes in providing high-quality lab equipment, including precision heating apparatus and temperature control systems, to ensure your experiments are accurate and reproducible.
Whether you are developing new cannabis products or conducting material analysis, our reliable equipment helps you maintain the exact temperatures needed for optimal results.
Contact our experts today to find the perfect heating solution for your laboratory's unique needs.
Visual Guide
Related Products
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
People Also Ask
- What is the function of laboratory autoclaves in SCWR research? Predict Material Compatibility and Corrosion Kinetics
- What role does an autoclave play in remediation experiments? Ensure Precision by Eliminating Biological Noise
- Why is a laboratory high-pressure autoclave sterilizer necessary? Ensure Accuracy in Antibacterial Research
- Are there any specific types of contamination that an autoclave cannot remove? Understanding the Limits of Steam
- What are the advantages of using an autoclave equipped with a stirring device for molten salt testing? Dynamic Accuracy


















