In sintering, the choice of atmosphere is a critical control parameter that dictates the chemical environment inside the furnace. The most common atmospheres range from inert gases like nitrogen and argon, to reducing gases like hydrogen and its blends, to specialized atmospheres like endothermic gas or a complete vacuum. Some materials, particularly certain ceramics, are even sintered in ambient air.
The purpose of a controlled sintering atmosphere is not merely to fill a space; it is to actively prevent undesirable chemical reactions like oxidation, remove surface contaminants, and, in some cases, intentionally alter the chemistry of the final part.

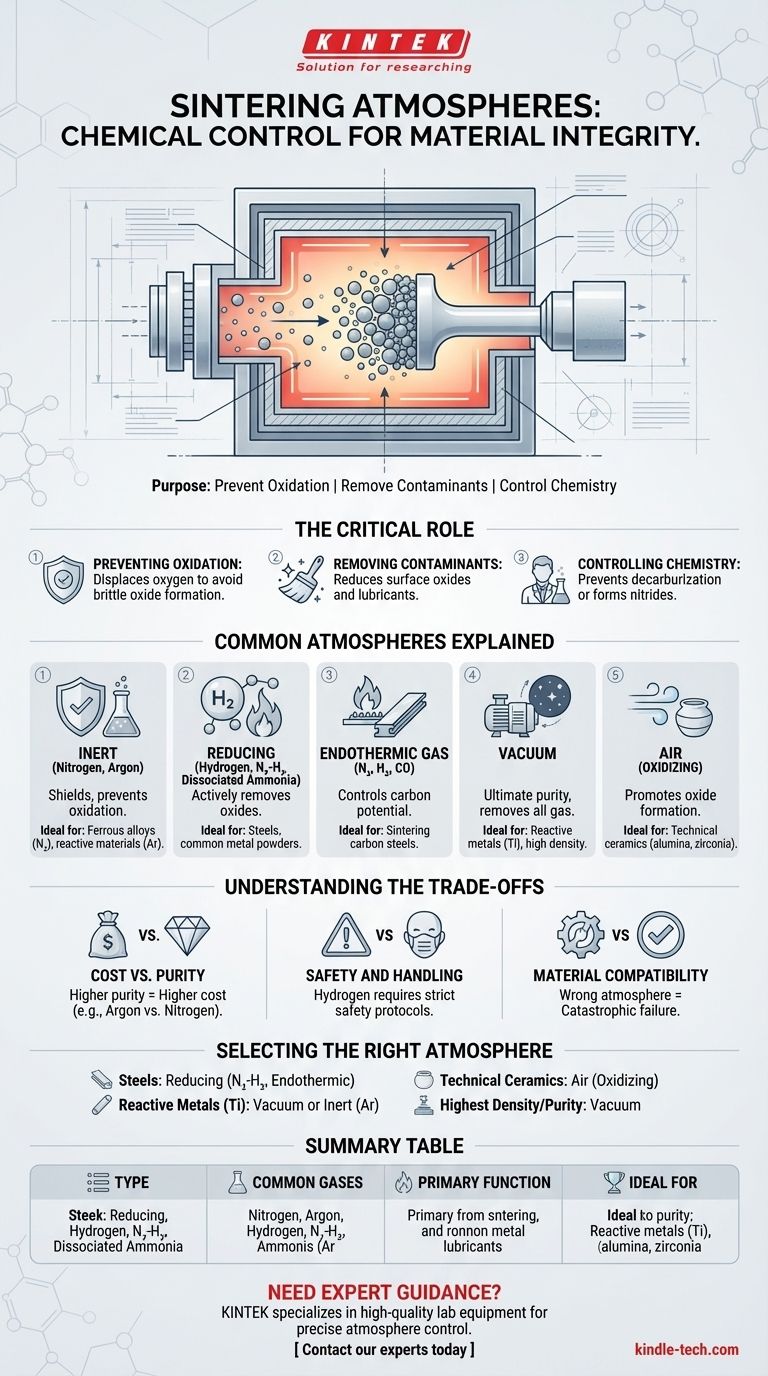
The Critical Role of the Atmosphere in Sintering
The extremely high temperatures required for sintering make materials highly susceptible to chemical reactions. The furnace atmosphere is the primary tool used to manage these reactions and ensure the integrity of the component.
Preventing Oxidation
The most fundamental job of a controlled atmosphere is to displace oxygen. At sintering temperatures, most metals will readily react with oxygen from the air, forming brittle and undesirable metal oxides on the particle surfaces. This prevents the particles from properly bonding and severely degrades the mechanical properties of the final part.
Removing Surface Contaminants
Before sintering, "green" parts often contain lubricants from the compaction process or may have a thin layer of existing surface oxides. A reducing atmosphere, such as one containing hydrogen, can chemically react with and remove these contaminants at elevated temperatures, ensuring clean, pure surfaces that can diffuse and bond effectively.
Controlling Material Chemistry
Some atmospheres are chosen to actively participate in the process. For example, an atmosphere with a controlled carbon potential (like endothermic gas) can prevent the loss of carbon from a steel part (decarburization). In other cases, a nitrogen-rich atmosphere can be used to intentionally form nitrides within the material, a process known as nitriding.
Common Sintering Atmospheres Explained
The atmosphere is chosen based on the material being processed, the desired final properties, and operational costs.
Inert Atmospheres (Nitrogen, Argon)
These gases are chemically neutral and serve as a simple "shielding" gas. Their primary function is to displace oxygen and prevent oxidation without reacting with the material itself. Nitrogen is a cost-effective and widely used option for many ferrous alloys, while argon is used for materials that might react with nitrogen at high temperatures.
Reducing Atmospheres (Hydrogen, Blends)
A reducing atmosphere actively removes oxygen. Hydrogen (H₂) is a powerful reducing agent, capable of stripping oxygen atoms from metal oxides. However, pure hydrogen is expensive and highly flammable.
For this reason, nitrogen-hydrogen (N₂-H₂) blends and dissociated ammonia (a mixture of hydrogen and nitrogen) are more common. They provide the reducing benefit of hydrogen in a safer and more economical mixture.
Endothermic Gas
Generated by reacting air and a hydrocarbon gas, endothermic gas (or "endo gas") is a carefully controlled mixture of nitrogen, hydrogen, and carbon monoxide. It is a reducing atmosphere primarily used for sintering steels, where its carbon potential can be precisely managed to match the carbon content of the alloy.
Vacuum
A vacuum is the ultimate "clean" atmosphere. By removing virtually all gas molecules, it eliminates any possibility of reaction with the material. Vacuum sintering is essential for highly reactive metals like titanium, refractory metals, and materials where the absolute highest purity and density are required.
Air (Oxidizing Atmosphere)
While often considered a contaminant for metals, air is the required atmosphere for sintering many technical ceramics. For materials like alumina or zirconia, the goal is to form a dense, stable oxide structure, making an oxygen-rich environment essential to the process.
Understanding the Trade-offs
The choice of atmosphere involves balancing material requirements with practical and economic constraints.
Cost vs. Purity
High-purity gases like argon and the equipment needed for high-vacuum sintering are significantly more expensive than running a furnace with a nitrogen-based atmosphere. The cost must be justified by the material's requirements.
Safety and Handling
Hydrogen is extremely flammable, requiring specialized safety protocols, ventilation, and monitoring. This is a major reason why nitrogen-hydrogen blends with low H₂ concentrations are preferred for many applications.
Material Compatibility
Using the wrong atmosphere can be catastrophic. A reducing atmosphere will ruin a ceramic that needs to be an oxide. A nitrogen-rich atmosphere can form unwanted nitrides in certain sensitive alloys. The atmosphere's chemistry must be perfectly matched to the material's chemistry.
Selecting the Right Atmosphere for Your Material
Your choice should be driven by the specific material you are working with and your end goal.
- If your primary focus is sintering common ferrous alloys (steels): A cost-effective reducing atmosphere like a nitrogen-hydrogen blend or an endothermic gas is typically the best choice.
- If your primary focus is sintering reactive metals (titanium, niobium) or cemented carbides: A high-purity inert gas like argon or, more commonly, a vacuum is necessary to prevent contamination.
- If your primary focus is sintering technical ceramics (alumina, zirconia): Air is often the correct choice to ensure the formation of a fully dense, stable oxide structure.
- If your primary focus is achieving the highest possible density and purity for any material: A vacuum provides the cleanest possible environment by removing all potential atmospheric reactants.
Ultimately, controlling the atmosphere is controlling the chemistry, which is the key to successful sintering.
Summary Table:
| Atmosphere Type | Common Gases/Environment | Primary Function | Ideal For |
|---|---|---|---|
| Inert | Nitrogen, Argon | Prevents oxidation by shielding | Ferrous alloys, materials sensitive to reaction |
| Reducing | Hydrogen, N₂-H₂ blends | Removes oxides and surface contaminants | Steels, common metal powders |
| Endothermic Gas | N₂, H₂, CO mixture | Controls carbon potential in steel | Sintering of carbon steels |
| Vacuum | Near-total gas removal | Eliminates all gas reactions for high purity | Reactive metals (titanium), high-density needs |
| Air (Oxidizing) | Ambient air | Promotes oxide formation for stability | Technical ceramics (alumina, zirconia) |
Need expert guidance on selecting the perfect sintering atmosphere for your materials? KINTEK specializes in providing high-quality lab equipment and consumables tailored to your laboratory's sintering needs. Whether you're working with reactive metals, ceramics, or standard alloys, our solutions ensure precise atmosphere control for optimal results. Contact our experts today to discuss how we can enhance your sintering process and improve your final product quality.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- When would you need to use a controlled atmosphere? Prevent Contamination and Control Reactions
- Why is a hydrogen atmosphere furnace necessary for W-Cu composite? Unlock Superior Infiltration and Density
- Why must a hydrogen-reducing atmosphere be maintained for tungsten annealing? Ensure Purity in High-Temp Processing
- What are the effects of hydrogen (H2) in a controlled furnace environment? Mastering Reduction and Risk
- What are hydrogen furnaces used for? Achieve Purity and Speed in High-Temperature Processing



















