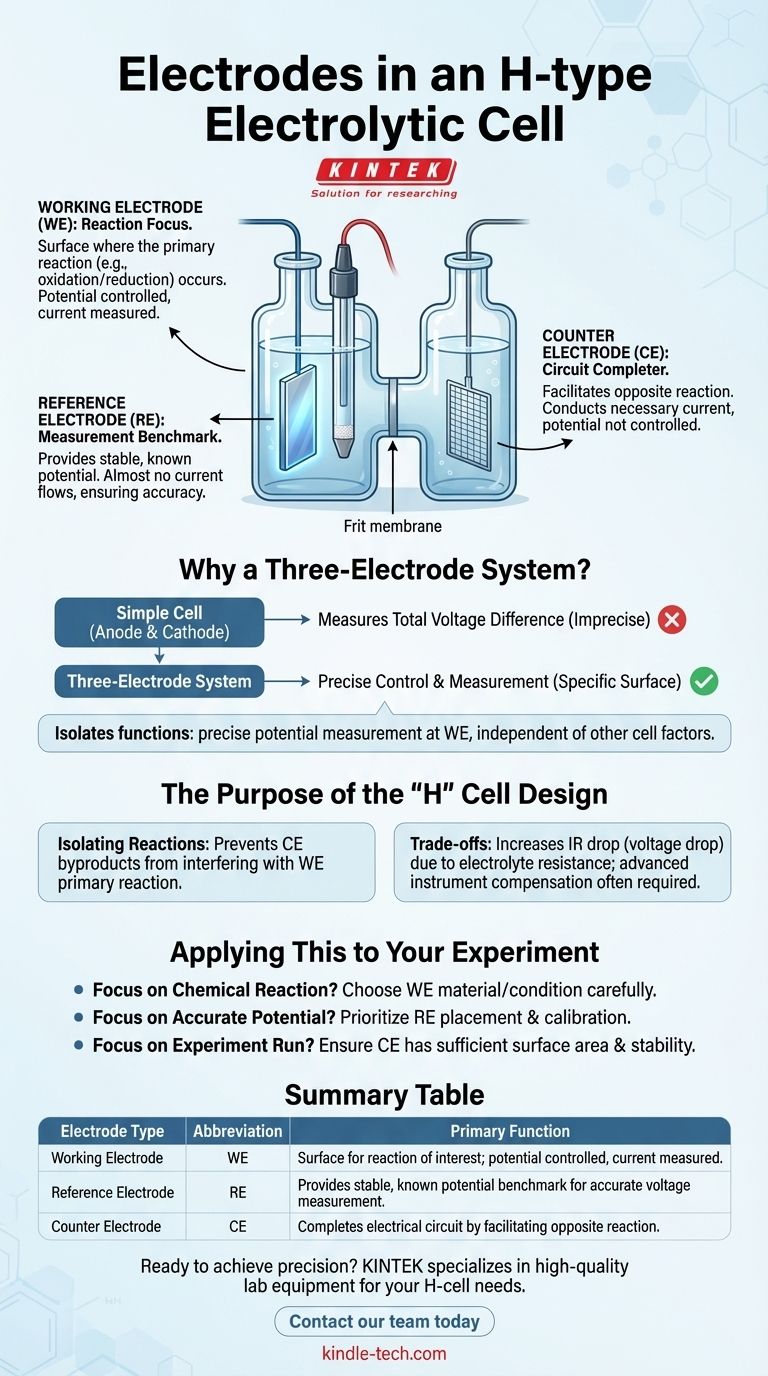
In short, an H-type electrolytic cell uses a three-electrode system to perform electrochemical analysis with high precision. These three distinct components are the working electrode (WE), the counter electrode (CE), and the reference electrode (RE). Each plays a specialized and critical role in controlling the experiment and accurately measuring the results.
The core principle is the separation of duties. While a simple cell combines the functions of current flow and voltage measurement into two electrodes, a three-electrode system isolates them. This allows an experimenter to precisely control and measure the voltage at the electrode where the reaction of interest is happening, independent of other factors in the cell.

Why a Three-Electrode System is the Standard
The goal of most electrochemical experiments is to study the reaction occurring at a specific surface. A simple two-electrode setup (anode and cathode) is insufficient for this because you can only measure the total voltage difference across the entire cell. You cannot know the precise potential at the single electrode surface you care about.
The Role of the Working Electrode (WE)
The working electrode is the focal point of the experiment. It is the surface where the electrochemical reaction you are investigating—be it oxidation or reduction—takes place.
Its potential is actively controlled by an instrument called a potentiostat, and the resulting current is measured. The material and preparation of the WE are critical variables in the experiment.
The Role of the Counter Electrode (CE)
The counter electrode, also known as the auxiliary electrode, has one primary job: to complete the electrical circuit. It conducts the electricity needed to support the reaction happening at the working electrode.
The CE simply provides a surface for the opposite reaction to occur (e.g., oxidation if the WE is undergoing reduction). Its own potential is not measured or controlled, and its material is chosen to be inert and capable of handling the required current without interfering with the main experiment.
The Critical Role of the Reference Electrode (RE)
The reference electrode is the key to accurate measurement. It acts as a stable, fixed benchmark with a known, unchanging potential.
A high-impedance voltmeter measures the potential difference between the working electrode and this stable reference electrode. Critically, almost no current flows through the reference electrode. This ensures its potential remains constant, providing a reliable zero point against which the working electrode's potential can be accurately measured.
The Purpose of the "H" Cell Design
The physical shape of the H-type cell is as important as the electrodes within it. The design physically separates the two halves of the cell to prevent unwanted interactions.
Isolating Reactions
The H-cell consists of two compartments, often joined by a glass frit or a semi-permeable membrane. The working and reference electrodes are placed in one compartment, while the counter electrode is placed in the other.
This separation prevents byproducts generated at the counter electrode from migrating over and contaminating or interfering with the primary reaction being studied at the working electrode.
Understanding the Trade-offs
The separation, while beneficial, is not without its costs. The electrolyte solution has its own resistance, and separating the electrodes increases this distance.
This resistance can cause a voltage drop (known as IR drop) between the reference and working electrodes, which can slightly skew the applied potential. Advanced instruments can often compensate for this, but it is a fundamental physical limitation to be aware of. The setup is also inherently more complex and costly than a simple beaker cell.
How to Apply This to Your Experiment
Understanding these components allows you to properly design and interpret your results. Your experimental goal dictates which electrode you should focus on.
- If your primary focus is studying a specific chemical reaction: The working electrode is your surface of interest, and you must choose its material and condition carefully.
- If your primary focus is obtaining stable and accurate potential measurements: The reference electrode is the non-negotiable component, and its proper placement and calibration are critical.
- If your primary focus is ensuring the experiment can run: The counter electrode's role is to pass the necessary current, and it must have sufficient surface area and stability to do so without interfering.
Mastering the function of each electrode is the foundation for conducting meaningful and repeatable electrochemical analysis.
Summary Table:
| Electrode Type | Abbreviation | Primary Function |
|---|---|---|
| Working Electrode | WE | Surface where the reaction of interest occurs; its potential is controlled and current is measured. |
| Reference Electrode | RE | Provides a stable, known potential benchmark for accurate voltage measurement. |
| Counter Electrode | CE | Completes the electrical circuit by facilitating the opposite reaction. |
Ready to achieve precision in your electrochemical experiments?
The correct electrode setup is fundamental to reliable data. KINTEK specializes in high-quality lab equipment and consumables, including the precise components needed for your H-cell and other electrochemical applications. Our experts can help you select the right electrodes and equipment to ensure your lab's success.
Contact our team today to discuss your specific laboratory needs and discover how KINTEK can support your research with reliable solutions.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the general storage and handling precautions for the electrolysis cell? Protect Your Lab's Precision Equipment
- How should a double-layer water-bath electrolytic cell be operated? A Step-by-Step Guide for Reliable Results
- What is the correct procedure for post-use handling and cleaning of an all-PTFE electrolytic cell? Ensure Purity and Longevity
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What material is the body of the electrolysis cell made of? High Borosilicate Glass for Reliable Electrochemistry
- How should the products and waste from the electrolytic cell be handled after an experiment? A Safety and Maintenance Guide
- How should the experimental parameters be adjusted when using the H-type electrolytic cell? Expert Precision Guide
- What role does an electrolytic etching cell play in analyzing swaged Cr-Ni-Ti steel? Reveal Hidden Microstructures



















