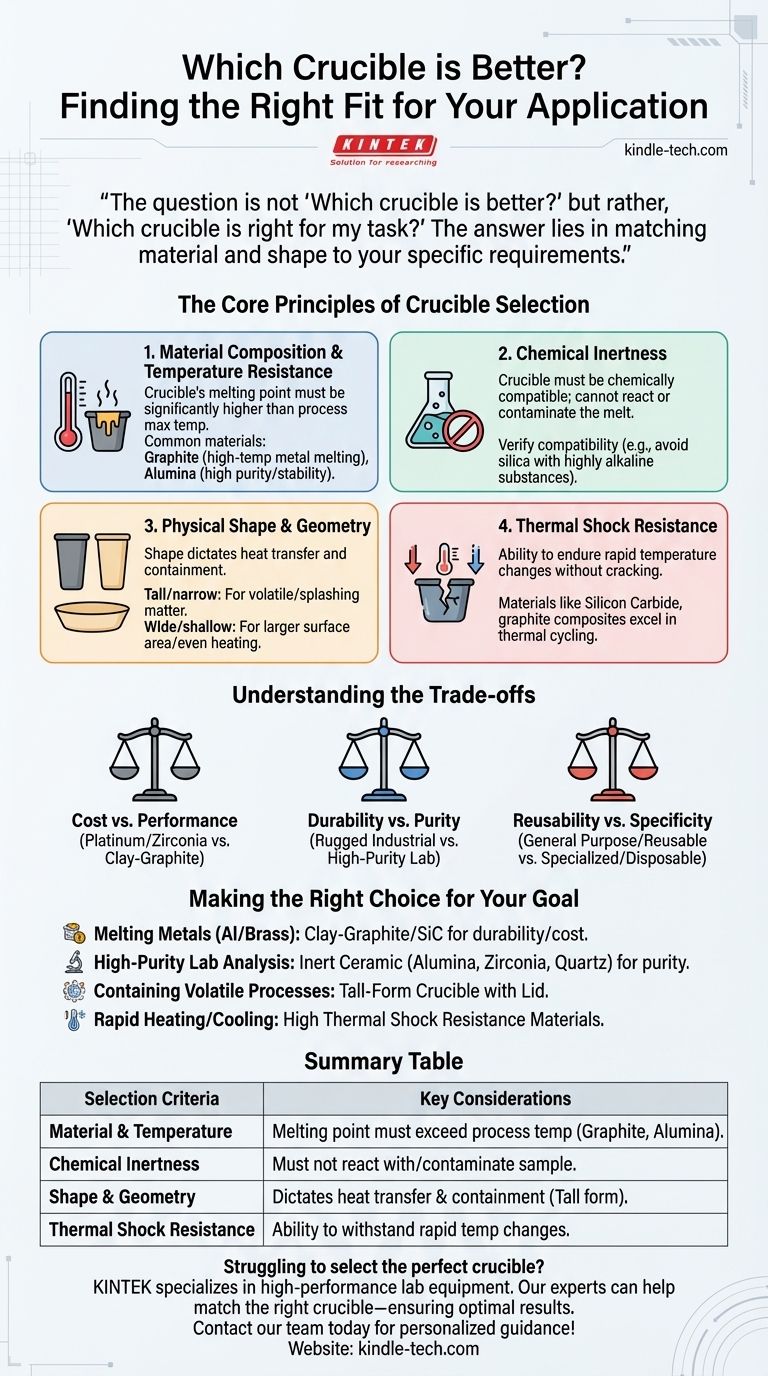
There is no single "better" crucible. The ideal choice is entirely dependent on your specific application. A crucible that performs perfectly for melting aluminum could fail catastrophically when used for a high-purity chemical analysis.
The question is not "Which crucible is better?" but rather, "Which crucible is right for my task?" The answer lies in carefully matching the crucible's material and shape to your specific requirements for temperature, chemical compatibility, and the physical process involved.

The Core Principles of Crucible Selection
Choosing the right crucible is a critical decision that impacts the safety, purity, and success of your work. The selection process revolves around four fundamental properties.
1. Material Composition and Temperature Resistance
The crucible's material is the single most important factor. Its melting point must be significantly higher than the maximum temperature of your process.
Common materials include graphite, excellent for high-temperature metal melting but unsuitable for oxidizing atmospheres, and alumina (ceramic), which offers high purity and stability but can be more brittle.
2. Chemical Inertness
A crucible must be chemically compatible with the materials it will hold. It cannot react with, dissolve in, or otherwise contaminate your melt.
For example, melting a highly alkaline substance in a silica-based crucible would cause the crucible to deteriorate and ruin the purity of the sample. Always verify the chemical compatibility between your material and the crucible.
3. Physical Shape and Geometry
The shape of the crucible is engineered for specific tasks. The geometry dictates how heat is transferred and how the contents behave.
A tall, narrow crucible (often called a "volatile matter" crucible) is designed to contain splashes and prevent the loss of materials that might sublime or spatter. In contrast, a wide, shallow form promotes a larger surface area for reactions or even heating.
4. Thermal Shock Resistance
Thermal shock is the stress a material endures when its temperature changes rapidly. A crucible with poor thermal shock resistance can crack or shatter if heated or cooled too quickly.
Materials like silicon carbide and certain graphite composites are known for their excellent thermal shock resistance, making them suitable for processes involving rapid temperature cycles.
Understanding the Trade-offs
No crucible is perfect for every situation. The selection process always involves balancing competing priorities.
Cost vs. Performance
High-performance crucibles made from materials like platinum or pure zirconia offer exceptional purity and temperature resistance but come at a significant cost. For many industrial applications, a less expensive clay-graphite crucible provides an acceptable balance of durability and price.
Durability vs. Purity
A rugged, thick-walled industrial crucible is built for durability over many cycles. However, its material composition may introduce minor impurities into the melt. Conversely, a high-purity laboratory crucible may be more fragile and have a shorter lifespan but will ensure the sample remains uncontaminated.
Reusability vs. Specificity
Some crucibles are designed to be general-purpose and reusable, while others are highly specialized or even disposable. A reusable crucible saves costs over time but requires careful cleaning to prevent cross-contamination between different batches.
Making the Right Choice for Your Goal
To select the correct crucible, start by defining your primary objective.
- If your primary focus is melting common metals like aluminum or brass: A clay-graphite or silicon carbide crucible offers the best combination of high-temperature resistance, durability, and cost-effectiveness.
- If your primary focus is high-purity lab analysis: Prioritize an inert ceramic crucible made of high-purity alumina, zirconia, or quartz to prevent sample contamination.
- If your primary focus is containing a volatile or reactive process: Select a tall-form crucible, often with a matching lid, to ensure the process is safely contained.
- If your primary focus is withstanding rapid heating and cooling: Choose a material known for excellent thermal shock resistance, such as a specialized graphite composite or silicon carbide.
Ultimately, selecting the right crucible is an investment in the safety, efficiency, and success of your work.
Summary Table:
| Selection Criteria | Key Considerations |
|---|---|
| Material & Temperature | Melting point must exceed process temperature (e.g., Graphite, Alumina). |
| Chemical Inertness | Must not react with or contaminate the sample. |
| Shape & Geometry | Dictates heat transfer and containment (e.g., tall form for volatile materials). |
| Thermal Shock Resistance | Ability to withstand rapid temperature changes without cracking. |
Struggling to select the perfect crucible for your lab's unique requirements? KINTEK specializes in high-performance lab equipment and consumables, including a comprehensive range of crucibles made from materials like graphite, alumina, and silicon carbide. Our experts can help you match the right crucible to your application—ensuring optimal temperature resistance, chemical inertness, and process efficiency. Contact our team today for personalized guidance and ensure the success of your next project!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- What is the best crucible for high temperatures? Match Your Material and Atmosphere for Success
- What are the three types of crucible furnaces? Lift-Out, Bale-Out, or Tilting?
- What is the primary function of a stainless steel crucible in studies involving liquid lead? Essential Lab Insights
- What material is used for induction furnace crucibles? Match Your Metal & Frequency for Optimal Melting
- What is the importance of high-purity ceramic crucibles in carbide melting experiments? Ensure High-Temperature Accuracy
- What are the advantages and disadvantages of a crucible furnace? A Guide to Simple, Versatile Melting
- What is the most durable crucible? Match the Right Crucible to Your Melting Application
- Why must high-purity ceramic crucibles be used for epoxy resin thermal analysis? Ensure Absolute Data Integrity



















