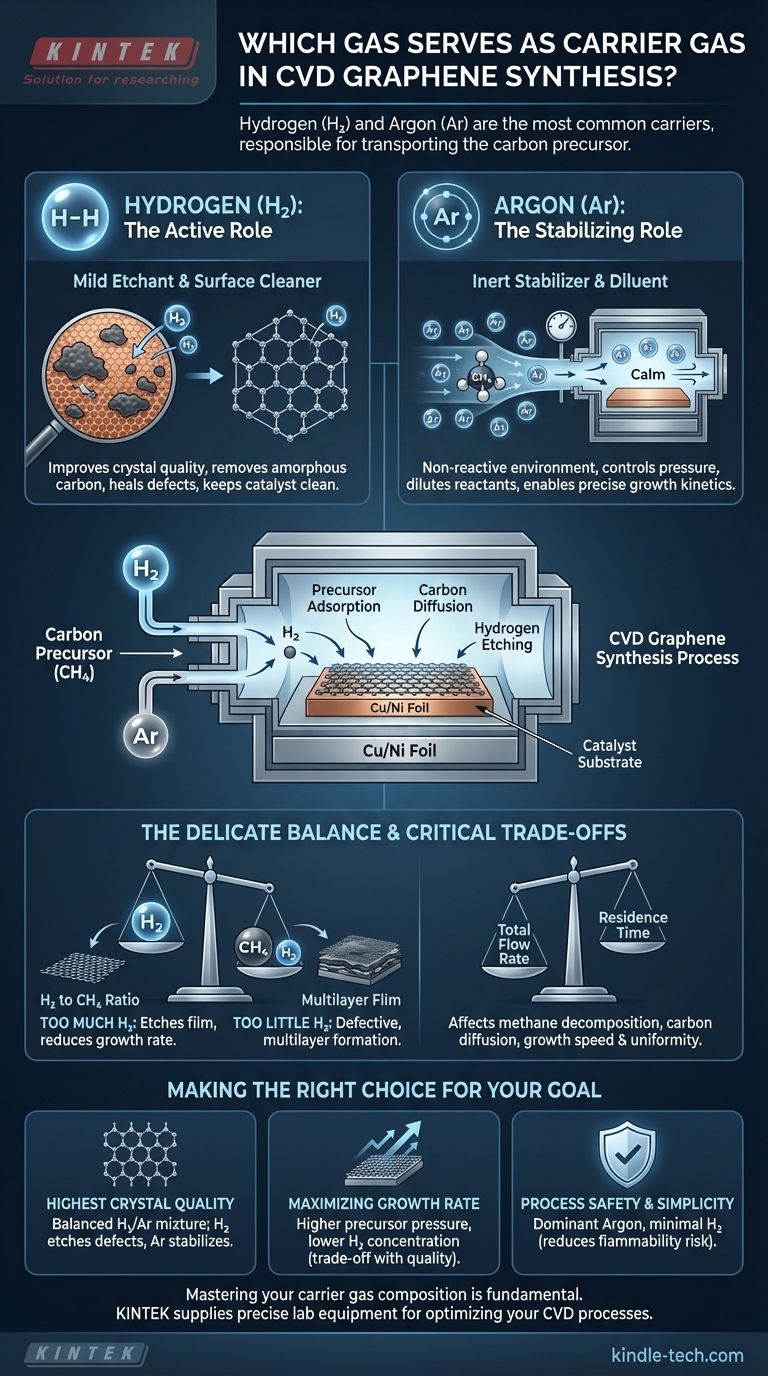
In chemical vapor deposition (CVD) for graphene, the most common carrier gases are hydrogen (H₂) and argon (Ar). These gases are responsible for transporting the carbon precursor gas, such as methane (CH₄), into the high-temperature reaction chamber and over the surface of the catalyst, which is typically a copper or nickel foil.
While their primary job is to transport the carbon source, the choice and ratio of carrier gases are critical control parameters. They actively shape the reaction environment, directly influencing the quality, growth rate, and final characteristics of the synthesized graphene.

The Core Functions of a Carrier Gas
The role of the carrier gas in the CVD process extends beyond simple transportation. It is fundamental to creating the precise conditions required for high-quality graphene growth.
Transporting the Precursor
The most basic function is to act as a delivery mechanism. The carrier gas mixes with the carbon source (methane) and flows through the system, ensuring a consistent supply of reactant molecules reaches the hot catalyst surface.
Maintaining Chamber Atmosphere
Carrier gases establish and maintain the required pressure and atmospheric conditions inside the furnace. Their flow rates are carefully controlled to purge the system of contaminants like oxygen before growth and to regulate the partial pressure of the reactants during the process.
Diluting the Reactants
The carrier gas dilutes the carbon precursor. This control is essential because the concentration of methane is a key factor in determining whether single-layer, bilayer, or multilayer graphene will form.
Why Hydrogen and Argon are Used Specifically
The selection of hydrogen and argon is not arbitrary; each gas serves a distinct and vital purpose in optimizing the synthesis.
The Active Role of Hydrogen (H₂)
Hydrogen is more than just a passive carrier. It acts as a mild etchant, which is crucial for improving the quality of the graphene film. It selectively removes less stable, amorphous carbon deposits and can help heal defects in the growing crystal lattice.
Furthermore, H₂ helps keep the surface of the copper or nickel catalyst clean and free of oxides, ensuring a pristine surface for graphene nucleation and growth.
The Stabilizing Role of Argon (Ar)
Argon is an inert noble gas. It does not react with the precursor, the catalyst, or the growing graphene. Its primary function is to provide a stable, non-reactive environment.
By using argon, researchers can dilute the reactive gases (methane and hydrogen) and gain precise control over their partial pressures, which directly influences the kinetics of the growth process.
Understanding the Trade-offs and Process Control
Achieving high-quality graphene consistently requires a deep understanding of how the gas mixture affects the outcome. The process is a delicate balance.
The Critical H₂ to CH₄ Ratio
The ratio of hydrogen to the methane precursor is arguably the most important parameter. Too much hydrogen can etch away the graphene film as it forms, severely reducing the growth rate.
Conversely, too little hydrogen can result in the formation of lower-quality, defective, or multilayer graphene due to the lack of its cleaning and etching effects.
Total Flow Rate and Residence Time
The total flow rate of all gases determines the residence time—how long the reactant molecules spend in the hot zone of the furnace. This affects the decomposition rate of the methane and the diffusion of carbon on the catalyst, impacting both growth speed and uniformity.
Making the Right Choice for Your Goal
The optimal gas mixture depends entirely on the desired outcome of the synthesis.
- If your primary focus is the highest crystal quality: A carefully controlled mixture of hydrogen and argon is often best, as H₂ etches defects while Ar provides a stable background pressure.
- If your primary focus is maximizing growth rate: A higher partial pressure of the carbon precursor with a lower H₂ concentration is typically used, though this often comes at the cost of reduced film quality.
- If your primary focus is process safety and simplicity: Using argon as the dominant carrier gas with only minimal hydrogen reduces the complexity and hazards associated with handling highly flammable H₂.
Mastering your carrier gas composition is a fundamental step toward achieving precise control over your graphene synthesis.
Summary Table:
| Gas | Primary Role | Key Impact on Graphene |
|---|---|---|
| Hydrogen (H₂) | Active etchant & surface cleaner | Improves crystal quality, removes defects |
| Argon (Ar) | Inert stabilizer & diluent | Controls pressure, enables precise growth kinetics |
| H₂/CH₄ Ratio | Critical process parameter | Determines layer number, growth rate, and film quality |
Achieve Precise Control Over Your Graphene Synthesis
Mastering the delicate balance of carrier gases is key to producing high-quality, consistent graphene films. The right lab equipment is essential for this level of control.
KINTEK specializes in supplying the precise lab equipment and consumables you need to optimize your CVD processes. Whether you are focusing on ultimate crystal quality, maximizing growth rate, or ensuring process safety, we have the solutions to support your research and development goals.
Ready to enhance your graphene synthesis? Contact our experts today to discuss how our products can help you achieve superior results.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
People Also Ask
- What is Ultra-High Vacuum CVD (UHVCVD)? Achieve Superior Purity in Advanced Material Deposition
- What is CVD in nanotechnology? The Key to Atomic-Level Material Fabrication
- Why is vacuum important in thin film coating? Achieve Purity and Control for Superior Film Quality
- How is deposition useful in IC fabrication? Building the Essential Layers for Microchips
- What are the pros and cons of physical vapor deposition? A Guide to PVD vs. CVD Coating
- What is sputtering in physics? A Guide to Atomic-Level Thin Film Deposition
- What is the synthesis of carbon nanotubes by chemical vapour deposition? Scalable Production for Your Lab
- How does a Chemical Vapor Deposition (CVD) reaction system modify nanomaterial-based packaging films? Enhance Durability



















