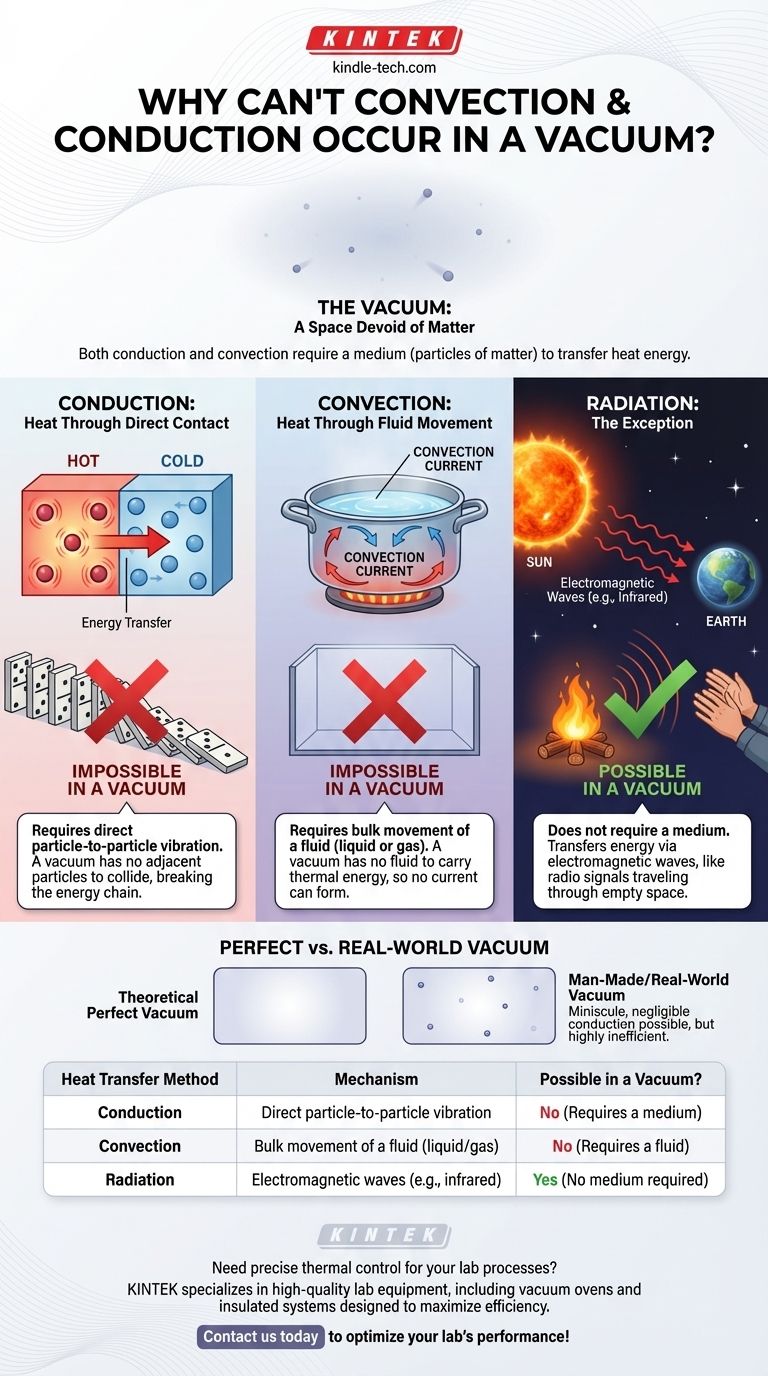
In short, both conduction and convection are impossible in a perfect vacuum because they fundamentally require a medium—particles of matter—to transfer heat energy. Conduction transfers heat through direct particle-to-particle vibrations, while convection transfers heat through the bulk movement of a fluid. Since a vacuum is, by definition, a space devoid of matter, there are no particles to vibrate or flow.
Heat has three methods of transfer, but only one can operate in a vacuum. Conduction and convection are like messengers that must run on a physical road (matter), whereas radiation is like a radio signal that travels through the empty air, requiring no road at all.

The Role of Matter in Heat Transfer
Heat is simply the transfer of thermal energy, which always moves from a hotter object to a colder one. The specific method of transfer, however, depends entirely on the environment between the objects.
Deconstructing Conduction: Heat Through Direct Contact
Conduction is the transfer of heat energy through direct contact. At the atomic level, the particles of a hotter object are vibrating more intensely than those of a colder object.
When these objects touch, the faster-vibrating particles of the hot object collide with the slower-vibrating particles of the cold object. This collision transfers kinetic energy, causing the colder particles to speed up (get hotter) and the hotter particles to slow down (get cooler).
Imagine a line of dominoes. Tipping the first one over (adding energy) causes a chain reaction that transfers that energy down the line.
Why Vacuum Halts Conduction
A vacuum is a space with no dominoes. There are no adjacent particles to collide with one another. Without a medium to propagate these vibrations, the chain of energy transfer is broken before it can even start.
This principle is the basis for vacuum-insulated thermoses. The vacuum layer between the inner and outer walls drastically reduces heat transfer via conduction and convection.
Deconstructing Convection: Heat Through Fluid Movement
Convection is the transfer of heat through the mass movement of fluids (liquids or gases). This process happens in a cycle.
When a fluid is heated, its particles gain energy, move faster, and spread apart, causing the fluid to become less dense. This less-dense, warmer fluid rises. Cooler, denser fluid from above then sinks to take its place, gets heated, and also rises. This circulation is called a convection current.
A pot of boiling water is a perfect example: hot water rises from the bottom as cooler water from the top sinks to be heated.
Why Vacuum Halts Convection
Convection is entirely dependent on having a fluid that can move and carry thermal energy with it. A vacuum contains no liquid or gas. With no fluid, there is nothing to form a current, and this mode of heat transfer is impossible.
The Exception: Radiation Thrives in a Vacuum
This raises a critical question: If the space between the Sun and Earth is a near-perfect vacuum, how does the Sun’s heat reach us? The answer is the third mode of heat transfer: thermal radiation.
The Mechanism of Radiation
Unlike conduction and convection, radiation does not require a medium. It transfers energy in the form of electromagnetic waves, primarily in the infrared spectrum.
Every object with a temperature above absolute zero emits these waves. The hotter the object, the more energy it radiates. These waves travel through space until they are absorbed by another object, transferring their energy and causing it to heat up.
This is how you feel the warmth of a campfire from a distance, even though the air between you might be cool. The fire's infrared radiation travels directly to you.
Understanding "Perfect" vs. "Real-World" Vacuums
It is important to distinguish between a theoretical perfect vacuum and the vacuums we can create or observe.
The Myth of the Perfect Vacuum
A "perfect" vacuum—a volume of space containing zero atoms or particles—is a theoretical concept. Even the vast emptiness of interstellar space contains a few hydrogen atoms per cubic meter.
Practical Implications
In a man-made vacuum, like in a thermos or a laboratory chamber, there are still some stray particles. This means a minuscule and often negligible amount of conduction can still occur. However, because the particles are so far apart, the transfer is incredibly inefficient and considered non-existent for most practical purposes.
How to Apply These Principles
Understanding the medium required for each form of heat transfer is key to controlling it in engineering and everyday life.
- If your primary focus is insulation (like in a thermos): Your goal is to stop all three transfer methods. A vacuum layer stops conduction and convection, and a reflective inner coating (like silvering) minimizes heat loss or gain from radiation.
- If your primary focus is heating a room: You are using convection. A radiator heats the air near it, which then rises and circulates throughout the room to distribute the warmth.
- If your primary focus is understanding space: You must recognize that radiation is the only way energy can travel across the vacuum of space, which is how stars heat planets.
Ultimately, whether heat can be transferred depends entirely on whether there is a physical pathway for the energy to follow.
Summary Table:
| Heat Transfer Method | Mechanism | Possible in a Vacuum? |
|---|---|---|
| Conduction | Direct particle-to-particle vibration | No (Requires a medium) |
| Convection | Bulk movement of a fluid (liquid/gas) | No (Requires a fluid) |
| Radiation | Electromagnetic waves (e.g., infrared) | Yes (No medium required) |
Need precise thermal control for your lab processes? Understanding heat transfer is fundamental to effective laboratory work. KINTEK specializes in high-quality lab equipment, including vacuum ovens and insulated systems designed to maximize efficiency by leveraging these principles. Let our experts help you select the right tools for your specific thermal management needs. Contact us today to optimize your lab's performance!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- How to vacuum out a furnace? A Step-by-Step Guide to Safe DIY Maintenance
- What materials are used in a vacuum furnace? Selecting the Right Hot Zone for Your Process
- What is a vacuum furnace used for? Unlock High-Purity Heat Treatment for Superior Materials
- How does argon and nitrogen cooling compare in vacuum furnaces? A Guide to Faster, Cheaper Quenching
- What is the structure of a vacuum furnace? A Guide to Its Core Components & Functions



















