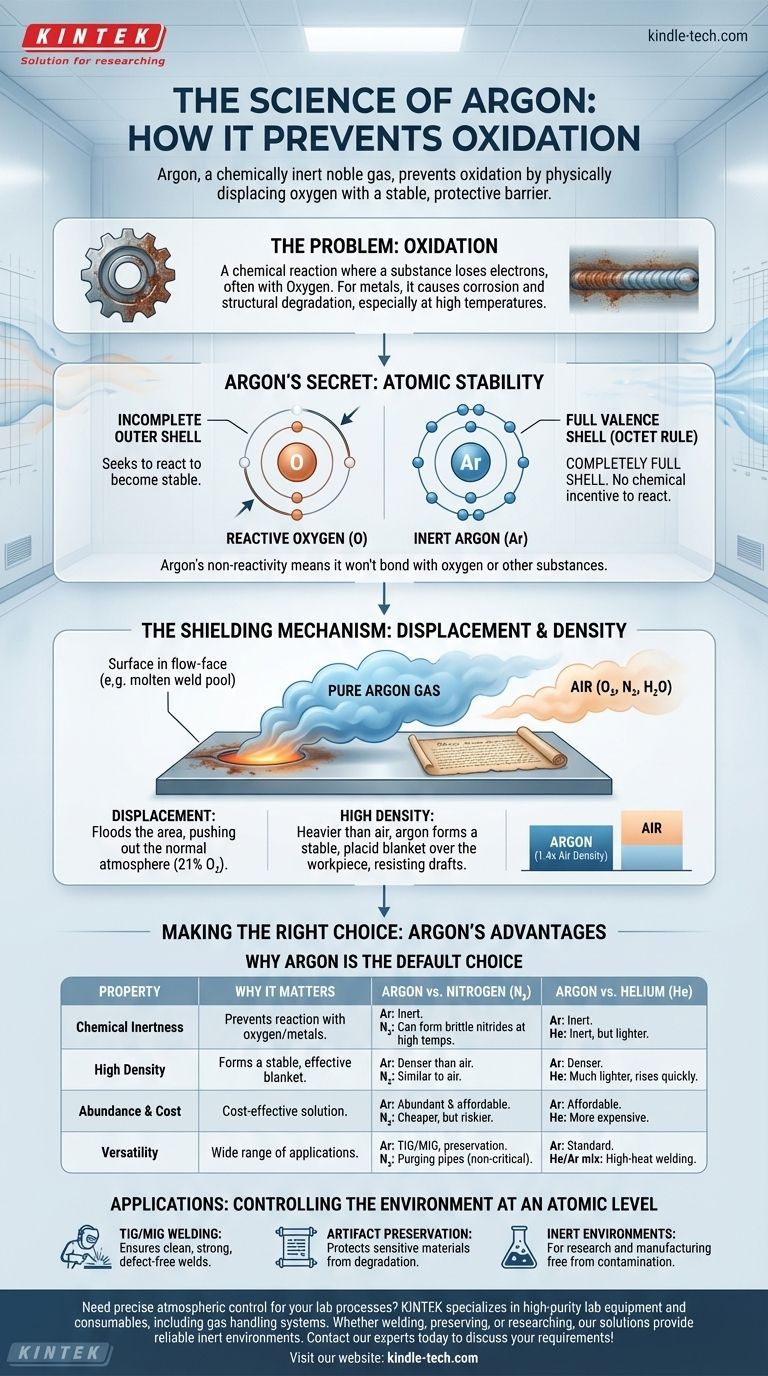
At its core, argon prevents oxidation because it is a chemically inert noble gas that physically displaces oxygen from a surface. Its atomic structure makes it unwilling to react with other elements, while its density allows it to form a stable, protective barrier that blocks oxygen and other atmospheric contaminants.
The key to understanding argon's power is its atomic stability. With a perfectly full outer shell of electrons, argon has no chemical incentive to react, making it an ideal, non-reactive shield against the highly reactive nature of oxygen.

The Chemistry of Oxidation
What Oxidation Is
Oxidation is a chemical reaction in which a substance loses electrons. While the name comes from oxygen, which is a very common agent for this process, it can happen with other elements as well.
For metals, this process is commonly known as corrosion or rust. The reaction degrades the material, weakening its structure and compromising its integrity.
Why It's a Problem
In processes like welding, the metal is heated to a molten state, making it extremely vulnerable to oxidation. Contact with oxygen at these temperatures can create oxides, leading to brittle welds, poor fusion, and catastrophic structural failure.
Likewise, sensitive items like historical documents or fine wine can be degraded by slow, long-term oxidation from the air.
Argon's Secret: A Full Valence Shell
The Definition of a Noble Gas
Argon belongs to Group 18 of the periodic table, known as the noble gases. This group, which also includes helium, neon, and xenon, is defined by its extreme lack of chemical reactivity.
The Power of the Octet Rule
The reason for this inertness lies in their electron configuration. Atoms strive for stability, which they typically achieve by having a full outer (or valence) shell of electrons, usually eight. This is known as the octet rule.
Think of an atom's valence shell as a dance card. Atoms like oxygen have an incomplete card and are aggressively looking for partners (electrons) to become stable. Argon, however, arrives with a completely full dance card.
Why This Makes Argon Non-Reactive
Because argon's outer electron shell is already full, it has no tendency to lose, gain, or share electrons with other atoms. It is chemically satisfied and stable.
This fundamental non-reactivity means it will not bond with oxygen, hot metal, or other substances, even under conditions of extreme heat or pressure. It simply exists as a neutral presence.
The Shielding Mechanism: Displacement and Protection
Creating an Oxygen-Free Zone
The primary way argon protects a surface is by displacement. By flooding an area with pure argon, you physically push out the normal atmosphere, which consists of about 21% oxygen and 78% nitrogen, plus water vapor.
This creates a localized, oxygen-free environment around the sensitive area, such as a weld pool or an ancient manuscript in a display case.
The Role of Density
Argon is approximately 1.4 times denser than air. This is a critical physical property for shielding applications.
Because it is heavier, argon tends to sink and form a stable, placid blanket over the workpiece. This provides consistent protection that is less easily disturbed by drafts compared to lighter gases like helium.
Understanding the Trade-offs
Why Not Just Use Nitrogen?
Nitrogen gas (N₂) is often used as a purging gas and is much cheaper than argon. However, it is not truly inert.
At the high temperatures of welding, nitrogen can react with certain metals, like titanium and some stainless steels, to form nitrides. These compounds can make the metal brittle, so argon is required for a chemically pure process.
Argon vs. Helium
Helium is the only other noble gas commonly used for shielding, but it has different properties. It is much lighter than air and has higher thermal conductivity.
The higher heat transfer of a helium-argon mix can be useful for welding very thick sections of conductive metals like aluminum. However, helium is more expensive and its low density means it rises quickly, requiring higher flow rates to maintain coverage.
The Cost-Effectiveness of Argon
Argon makes up nearly 1% of Earth's atmosphere, making it the most abundant and least expensive of all the noble gases. This combination of perfect inertness, ideal density, and affordability makes it the default choice for the vast majority of shielding applications.
Making the Right Choice for Your Application
Choosing a shielding gas requires matching its properties to your specific goal.
- If your primary focus is TIG or MIG welding most steels and aluminum: Argon is the industry standard due to its excellent arc stability, ideal density, and cost-effectiveness.
- If your primary focus is preserving sensitive artifacts or food products: Argon is superior because its total inertness and density create a permanent, non-reactive protective blanket.
- If your primary focus is high-speed or deep-penetration welding on thick, non-ferrous metals: A specialized argon/helium mixture may be necessary to leverage helium's higher thermal energy.
- If your primary focus is simply purging pipes or containers for non-critical applications: Nitrogen may be a more cost-effective choice, provided it won't react with the materials involved.
Ultimately, understanding the chemical stability of argon empowers you to control the environment at an atomic level.
Summary Table:
| Property | Why It Matters for Oxidation Prevention |
|---|---|
| Chemical Inertness | Argon does not react with oxygen or hot metals, even at high temperatures. |
| High Density | Heavier than air, it forms a stable blanket that displaces oxygen effectively. |
| Abundance & Cost | Makes up 1% of the atmosphere, offering a cost-effective shielding solution. |
| Versatility | Ideal for TIG/MIG welding, artifact preservation, and creating inert environments. |
Need precise atmospheric control for your lab processes? KINTEK specializes in high-purity lab equipment and consumables, including gas handling systems, to ensure your experiments and production are free from contamination. Whether you're welding, preserving sensitive materials, or conducting research, our solutions provide the reliable inert environments you need. Contact our experts today to discuss how we can support your specific laboratory requirements!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- What is an inert condition? A Guide to Preventing Fires and Explosions
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization



















