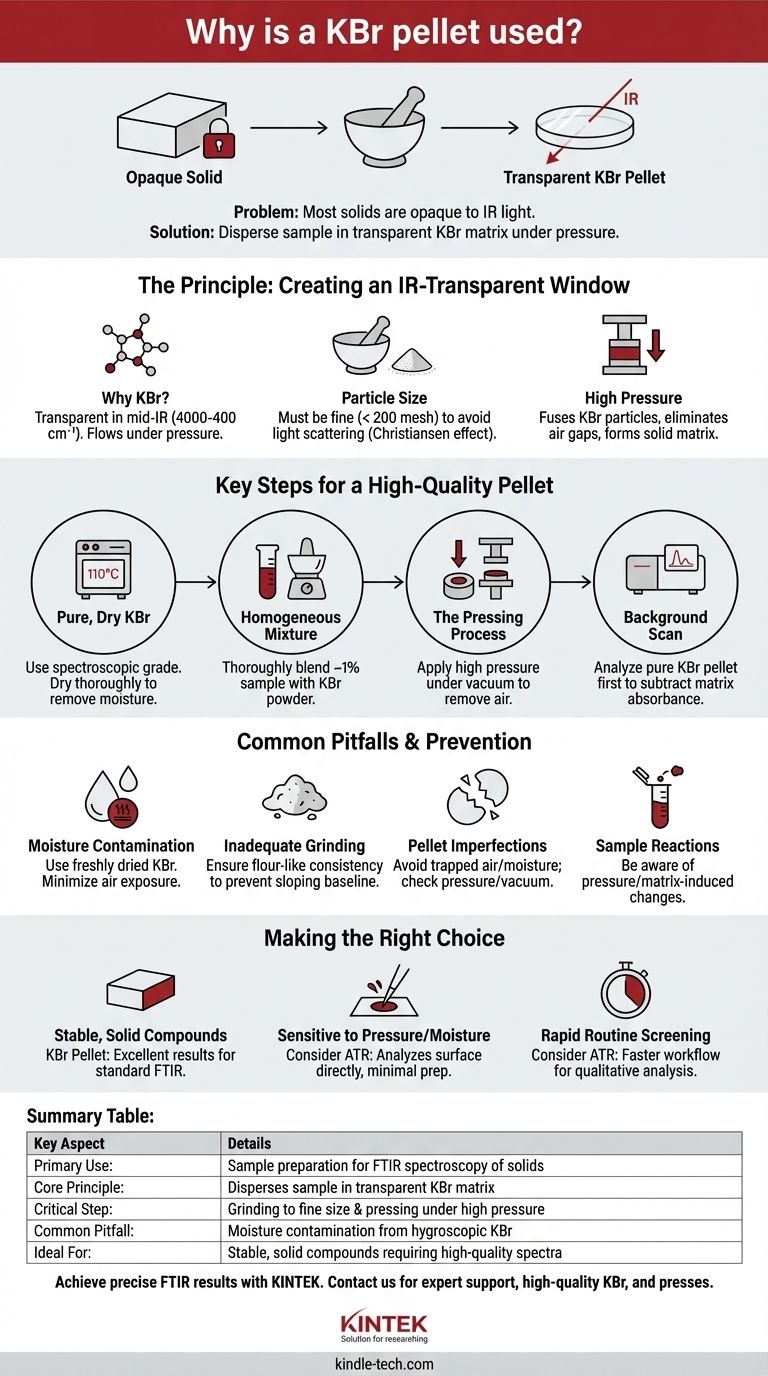
In Fourier-transform infrared (FTIR) spectroscopy, a potassium bromide (KBr) pellet is used to prepare solid samples for analysis. Because infrared light cannot pass through most solid materials in their bulk form, the sample is finely ground and dispersed within a KBr matrix, which is transparent to infrared radiation. Pressing this mixture under high pressure creates a thin, translucent disc that allows the spectrometer's IR beam to pass through the sample and measure its molecular vibrations.
The core challenge with solid samples is their opacity to infrared light. The KBr pellet technique solves this by finely dispersing the sample within a KBr matrix, which is transparent in the mid-IR region, effectively turning an opaque powder into a translucent "window" for analysis.

The Principle: Creating an Infrared-Transparent Window
The success of the KBr pellet method hinges on a few key physical principles that work together to allow for the analysis of otherwise intractable solid samples.
Why Potassium Bromide (KBr)?
Potassium bromide is the material of choice because it possesses two critical properties. First, it does not absorb light in the mid-infrared range (4000-400 cm⁻¹), which is where most organic and inorganic compounds show their characteristic molecular vibrations. Second, KBr is a soft, crystalline salt that flows and fuses under high pressure, forming a solid, glass-like disc.
The Role of Particle Size
A crucial step is to grind both the sample and the KBr powder into extremely fine particles (typically to a 200 mesh size). If the particles are too large, they will scatter the infrared light instead of transmitting it. This scattering effect, known as the Christiansen effect, results in a distorted, sloping baseline and makes the resulting spectrum difficult to interpret.
The Function of High Pressure
Once the sample and KBr are intimately mixed, they are placed in a die and subjected to several tons of pressure. This immense force causes the soft KBr particles to deform and fuse together, eliminating air gaps and trapping the finely dispersed sample particles within a solid, ordered matrix. The final product is a pellet that is mechanically stable and transparent enough for analysis.
Key Steps for a High-Quality Pellet
Achieving a clear, usable KBr pellet requires careful attention to detail throughout the preparation process. Each step is designed to mitigate a potential source of error.
Starting with Pure, Dry Material
The process must begin with spectroscopic grade KBr. Any impurities in the KBr will introduce their own absorbance peaks, contaminating the spectrum. Furthermore, KBr is highly hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong IR absorbance bands, so the KBr must be thoroughly dried in an oven (around 110 °C) before use to prevent these interfering peaks.
Achieving a Homogeneous Mixture
The sample must be thoroughly and evenly blended with the KBr powder, typically at a concentration of about 1% sample to KBr. If the mixture is not homogeneous, the concentration of the sample will vary across the pellet, leading to a non-representative and potentially non-reproducible spectrum.
The Pressing Process
The KBr mixture is loaded into a pellet die, and pressure is applied using a hydraulic press. This is often done under vacuum to remove trapped air and residual moisture. Insufficient vacuum or pressure will result in a cloudy, brittle pellet that scatters light and provides a poor-quality signal.
Running the Background Scan
Before analyzing the sample pellet, a background measurement must be performed using a pure KBr pellet. This allows the spectrometer to record and subtract any absorbance signals from the KBr matrix itself, atmospheric CO₂, or residual water vapor, ensuring the final spectrum shows only the absorbance of the sample.
Common Pitfalls and How to Avoid Them
While powerful, the KBr technique is sensitive to procedural errors that can degrade the quality of the data. Understanding these common issues is key to obtaining reliable results.
Moisture Contamination
This is the most frequent problem. Because KBr is so hygroscopic, exposure to ambient air can introduce significant water peaks into the spectrum. Always use freshly dried KBr and minimize the time the powder and pellet are exposed to the air.
Inadequate Grinding
If the sample or KBr is not ground finely enough, significant light scattering will occur. This manifests as a sloping baseline that can obscure weaker absorbance peaks. Ensure the material has a flour-like consistency before pressing.
Pellet Imperfections
A pellet that is cloudy, opaque, or easily broken is a sign of a flawed process. This is typically caused by trapped air or moisture from an inadequate vacuum, insufficient pressure, or KBr that was not properly dried.
Sample Reactions
In some cases, the high pressure applied during pellet formation can induce a chemical or physical change in the sample. Furthermore, some samples can react with the ionic KBr matrix, altering their chemical structure and leading to a spectrum that does not represent the original material.
Making the Right Choice for Your Goal
The KBr pellet method is a classic and effective technique, but its suitability depends on your sample and analytical goals.
- If your primary focus is obtaining a high-quality spectrum from a stable, solid compound: The KBr pellet method is a reliable and standard technique that provides excellent results when performed correctly.
- If your primary focus is analyzing a sample that is sensitive to pressure or moisture: You should consider alternative methods like Attenuated Total Reflectance (ATR) spectroscopy, which analyzes the surface of a sample directly with minimal preparation.
- If your primary focus is rapid, routine screening of many solid samples: The KBr method can be time-consuming; modern ATR accessories often provide a much faster workflow for qualitative analysis.
Mastering the KBr pellet technique is a fundamental skill that transforms opaque solids into analyzable samples, unlocking a wealth of molecular information.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Use | Sample preparation for FTIR spectroscopy of solids |
| Core Principle | Disperses sample in a transparent KBr matrix |
| Critical Step | Grinding to fine particle size and pressing under high pressure |
| Common Pitfall | Moisture contamination from hygroscopic KBr |
| Ideal For | Stable, solid compounds requiring high-quality spectra |
Achieve precise and reliable FTIR results with expert sample preparation support. KINTEK specializes in providing high-quality laboratory equipment and consumables, including spectroscopic grade KBr and reliable pellet presses, to meet the stringent demands of your laboratory. Ensure your sample preparation is flawless—contact our experts today to discuss your specific FTIR needs and discover the right solutions for accurate molecular analysis.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What is the purpose of using a laboratory hydraulic press for cold pre-pressing? Optimize Your Composite Synthesis
- What are the specifications of XRF? A Guide to Elemental Analysis from Mg to U
- What are the two applications of a hydraulic press? From Industrial Forging to Lab Analysis
- How does a laboratory hydraulic press ensure process consistency? Achieve Reliable CR2032 Coin Cell Assembly
- Why are laboratory hydraulic presses necessary for halide solid-state batteries? Achieve Optimal Electrolyte Density
- What role does a laboratory hydraulic press play in testing conductivity? Enhancing Nanoparticle Powder Analysis
- Do hydraulics need to warm up? Protect Your Equipment from Cold-Start Damage
- How many types of hydraulic presses are there? A Guide to Frame Designs for Your Application



















