In short, debinding is the essential process of removing the temporary "binder" material from a molded part. This step acts as a critical bridge in manufacturing methods like metal injection molding (MIM) and certain types of 3D printing. It purifies the component, leaving behind only the primary material (like metal or ceramic powder) and preparing it for the final strengthening and densification stage known as sintering.
Debinding is fundamentally a process of controlled subtraction. Its success determines whether a part can survive the final heating stage to become dense and strong, or if it will fail due to internal defects like cracks, voids, or distortion.

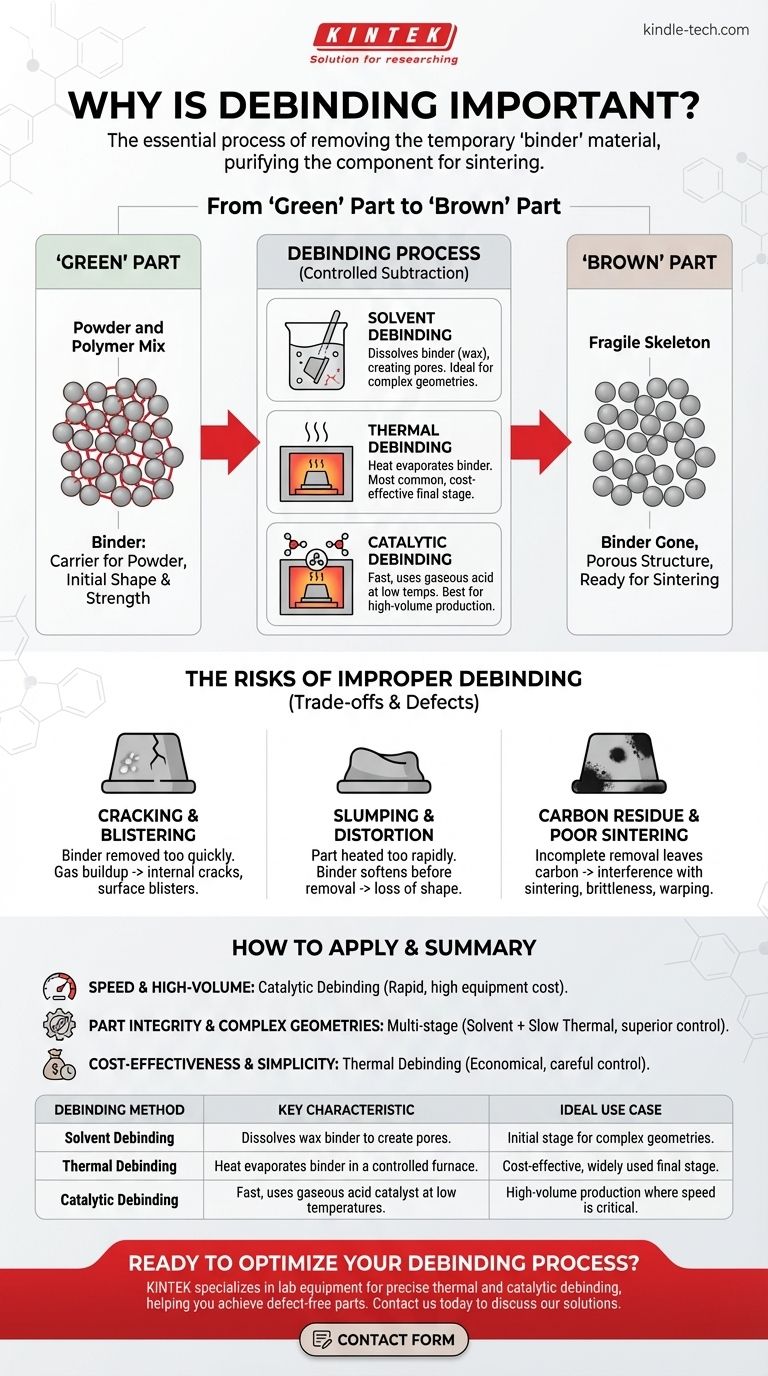
From "Green" Part to "Brown" Part
To understand why debinding is so critical, you must first understand the role of the binder it removes.
The "Green" Part: A Powder and Polymer Mix
The initial component formed by molding or printing is called a "green" part. This part is not made of pure metal or ceramic.
Instead, it's a precise mixture of the final material powder and a binder system. The binder is typically a blend of waxes and polymers that acts as a temporary scaffold.
The Binder's Purpose: A Carrier for the Powder
The binder is crucial for the initial shaping process. It liquefies under heat, allowing the powder to be injected into a mold and hold a complex shape with high precision.
Without the binder, the raw powder could not be molded. The binder provides the "green" part with its initial shape and handling strength.
The "Brown" Part: A Fragile Skeleton
After the debinding process is complete, the component is known as a "brown" part.
At this stage, the binder is gone, leaving a fragile, porous structure of metal or ceramic particles held together by friction. It is extremely brittle but now ready for the final sintering furnace.
The Mechanics of Debinding: How Binders Are Removed
Debinding is not a single method but a category of processes. The chosen method depends on the binder material, part geometry, and production goals.
Solvent Debinding
In this process, the "green" part is submerged in a liquid solvent. This solvent dissolves a portion of the binder—typically the wax component—creating a network of open pores throughout the part.
This allows the remaining polymer binder to be removed more easily in a subsequent step.
Thermal Debinding
Thermal debinding is the most common method, often used as a final stage after solvent debinding. The part is heated slowly in a controlled-atmosphere furnace.
The heat carefully breaks down and evaporates the remaining binder. The rate of heating must be precise to allow the binder gases to escape without building up pressure.
Catalytic Debinding
This is the fastest method. "Green" parts are placed in a furnace with a gaseous acid catalyst, such as nitric acid.
The catalyst rapidly breaks down the primary polymer binder (like polyacetal) at low temperatures, allowing it to be removed in a fraction of the time required for thermal debinding.
Understanding the Trade-offs: The Risks of Improper Debinding
Debinding is a delicate operation. If performed incorrectly, it will create defects that are impossible to fix in the final sintering stage.
The Risk of Cracking and Blistering
If the binder is removed too quickly, the gases it forms can become trapped inside the part. As pressure builds, it can cause blisters on the surface or generate internal cracks that severely compromise the part's strength.
The Problem of Slumping and Distortion
If a part is heated too rapidly during thermal debinding, the binder can soften before it has been sufficiently removed. Without its internal support structure, the part can sag under its own weight, leading to a loss of dimensional accuracy.
The Issue of Carbon Residue
Incomplete thermal debinding can leave behind carbon residue from the polymer. This carbon can interfere with the final material's chemistry during sintering, leading to brittleness and poor mechanical performance.
The Impact on Sintering
A well-debound part has a uniform network of pores. This allows it to shrink predictably and evenly during sintering. A poorly debound part with non-uniform density will warp, crack, or fail to reach its target density, resulting in a rejected component.
How to Apply This to Your Project
Your choice of debinding strategy should align directly with your manufacturing priorities.
- If your primary focus is speed and high-volume production: Catalytic debinding is often the best choice for its rapid cycle times, but it requires a specific binder system and has higher equipment costs.
- If your primary focus is part integrity for complex geometries: A multi-stage process, like solvent debinding followed by a slow thermal cycle, offers superior control and minimizes stress, reducing the risk of defects in intricate parts.
- If your primary focus is cost-effectiveness and process simplicity: A single, carefully controlled thermal debinding cycle can be the most economical solution, especially when the absolute fastest turnaround is not the main driver.
Mastering the debinding process is mastering the foundation upon which high-quality, high-performance parts are built.
Summary Table:
| Debinding Method | Key Characteristic | Ideal Use Case |
|---|---|---|
| Solvent Debinding | Dissolves wax binder to create pores. | Initial stage for complex geometries. |
| Thermal Debinding | Heat evaporates binder in a controlled furnace. | Cost-effective, widely used final stage. |
| Catalytic Debinding | Fast, uses gaseous acid catalyst at low temperatures. | High-volume production where speed is critical. |
Ready to Optimize Your Debinding Process?
The right debinding strategy is the foundation of successful MIM and 3D printing. KINTEK specializes in the lab equipment and consumables needed for precise thermal and catalytic debinding, helping you achieve defect-free parts with superior mechanical properties.
Contact us today using the form below to discuss how our solutions can enhance your manufacturing quality and efficiency.
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Dental Porcelain Sintering Furnace
People Also Ask
- How much sample is needed for IR? Optimize Your Analysis with Minimal Material
- Can sintered parts be machined? How to Overcome the Challenges of Porosity
- How energy-intensive are ULT freezers and what are their operating costs? Discover the True Cost of Ultra-Low Temperature Storage
- What are the advantages of press forging over drop forging? Achieve Superior Internal Integrity for Critical Components
- Why is pyrolysis of solid waste important? Transform Waste into Fuel and Valuable Resources
- What is sputtering techniques of thin film deposition? Achieve Superior Coatings with Material Versatility
- What is the difference between laser melting and sintering? A Guide to Particle Fusion Methods
- Why is it necessary to configure drying equipment before TSA? Boost CO2 Capture Efficiency and Adsorbent Life



















