At its core, electrolytic deposition is a process used to apply a thin, functional metal coating onto the surface of an object. It is widely employed across industries to achieve a specific outcome that the base material cannot provide on its own, such as improving corrosion resistance, enhancing aesthetic appeal, increasing hardness, or modifying electrical conductivity.
While often seen as a simple "plating" technique, the true value of electrolytic deposition lies in its precision. It is a highly controllable and cost-effective method for engineering specific surface properties, fundamentally changing how a component performs in its environment.
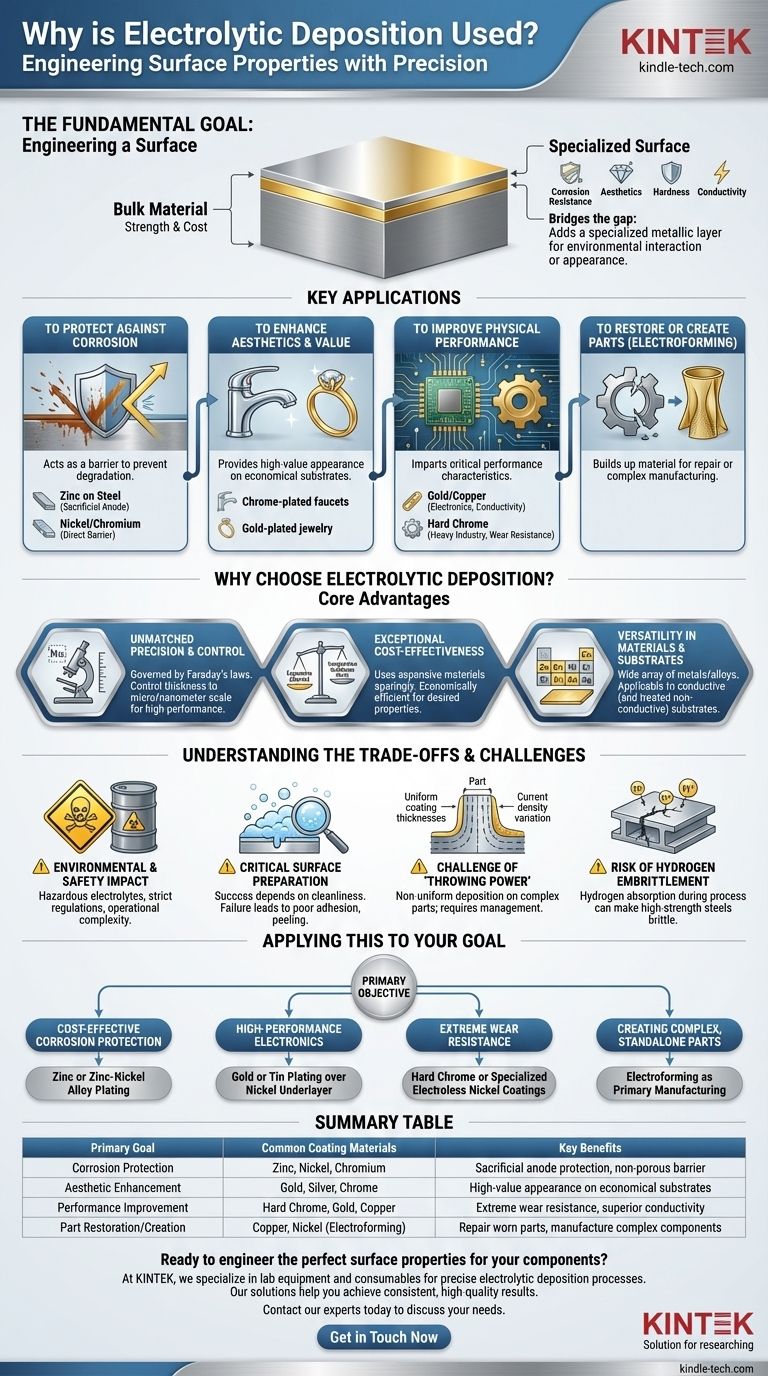
The Fundamental Goal: Engineering a Surface
Electrolytic deposition solves a common engineering problem: the ideal material for the bulk of a part (for strength or cost) is rarely the ideal material for its surface (for environmental interaction or appearance). The process bridges this gap by adding a specialized metallic layer.
To Protect Against Corrosion
One of the most common uses is to protect a reactive base metal, like steel, from environmental degradation. The deposited layer acts as a barrier.
For example, a thin layer of zinc is often deposited onto steel bolts and fasteners. The zinc acts as a sacrificial anode, corroding preferentially to protect the steel underneath. Nickel and chromium layers provide a more direct, non-porous barrier against moisture and oxygen.
To Enhance Aesthetics and Value
The process is responsible for the bright, reflective finishes on countless consumer products. It provides an appearance of high value on a more economical substrate.
Think of chrome-plated faucets, silver-plated tableware, or gold-plated jewelry. In these cases, a less expensive base metal like brass or steel provides the structure, while a micro-thin layer of a precious or decorative metal provides the desired look and feel.
To Improve Physical Performance
Beyond appearance, deposited layers can impart critical performance characteristics. The properties of the coating are often distinct from the bulk material.
In electronics, gold and copper are deposited onto connectors and circuit boards to ensure excellent electrical conductivity and prevent oxidation. In heavy industry, hard chrome is applied to pistons, rollers, and hydraulic cylinders to create an incredibly hard, low-friction, and wear-resistant surface.
To Restore or Create Parts
Electrolytic deposition can also be used to build up material. This process, often called electroforming, can repair worn or incorrectly machined parts by adding material back to critical dimensions.
Furthermore, it can be used as a primary manufacturing method to create complex, thin-walled metal objects, such as wave guides or bellows, that would be difficult or impossible to produce with traditional machining.
Why Choose Electrolytic Deposition? The Core Advantages
While other coating methods exist, electrolytic deposition remains a dominant process due to a unique combination of control, cost, and versatility.
Unmatched Precision and Control
The process is governed by Faraday's laws of electrolysis, which means the amount of metal deposited is directly proportional to the electrical charge passed through the system.
This relationship allows for extremely precise control over coating thickness, often down to the micrometer or even nanometer scale. This level of precision is essential for high-performance applications in aerospace and electronics.
Exceptional Cost-Effectiveness
Electrolytic deposition allows engineers to use expensive materials sparingly. Applying a 10-micrometer layer of gold to a connector is vastly cheaper than making the entire connector from solid gold.
This principle of using a strong, inexpensive substrate with a thin, high-performance surface layer makes it one of the most economically efficient ways to achieve desired material properties.
Versatility in Materials and Substrates
A vast array of metals and alloys can be deposited, including zinc, copper, nickel, chromium, tin, gold, silver, and platinum.
The process can be applied to any conductive substrate. With special pretreatment steps to create a conductive seed layer, it can even be used to plate non-conductive materials like plastics and ceramics.
Understanding the Trade-offs and Challenges
Despite its advantages, electrolytic deposition is a complex process with significant challenges that require expert management. Being aware of these is critical for successful implementation.
Environmental and Safety Impact
Many electroplating solutions, or electrolytes, contain hazardous materials. Cyanide baths, heavy metals like cadmium and hexavalent chromium, and strong acids pose significant risks to workers and the environment.
Strict regulations govern the handling, ventilation, and treatment of these chemicals and the waste they produce, adding significant operational complexity and cost.
The Critical Role of Surface Preparation
The success of electrolytic deposition is overwhelmingly dependent on the cleanliness and preparation of the substrate. The surface must be completely free of oils, oxides, and other contaminants.
Any failure in the multi-step cleaning and activation process will result in poor adhesion, causing the coating to blister, peel, or flake off in service.
The Challenge of "Throwing Power"
The electric field that drives deposition is not uniform across a complex part. Higher current density occurs on sharp external corners, leading to thicker deposits, while deep recesses or holes receive lower current density and thus a thinner coating.
This phenomenon, known as "throwing power," must be managed through careful electrolyte chemistry, part orientation, and the use of auxiliary anodes to achieve a uniform coating on geometrically complex components.
The Risk of Hydrogen Embrittlement
During deposition, hydrogen atoms can be generated and subsequently diffuse into the crystal structure of high-strength steels. This can make the metal brittle and prone to sudden failure under load.
This risk, known as hydrogen embrittlement, is a serious concern in aerospace and automotive applications. It must be mitigated by a post-plating baking process that drives the trapped hydrogen out of the material.
Applying This to Your Goal
To select the right approach, you must first define your primary objective for the surface.
- If your primary focus is cost-effective corrosion protection: Consider zinc or zinc-nickel alloy plating for sacrificial protection on steel components.
- If your primary focus is high-performance electronics: Use gold or tin plating over a nickel underlayer for superior conductivity and solderability.
- If your primary focus is extreme wear resistance for industrial parts: Look to hard chrome or specialized electroless nickel coatings for their exceptional hardness and low friction.
- If your primary focus is creating complex, standalone metal parts: Investigate electroforming as a primary manufacturing process, not just a coating.
By understanding these principles, you can leverage electrolytic deposition not just as a finishing step, but as a precise surface engineering tool.
Summary Table:
| Primary Goal | Common Coating Materials | Key Benefits |
|---|---|---|
| Corrosion Protection | Zinc, Nickel, Chromium | Sacrificial anode protection, non-porous barrier |
| Aesthetic Enhancement | Gold, Silver, Chrome | High-value appearance on economical substrates |
| Performance Improvement | Hard Chrome, Gold, Copper | Extreme wear resistance, superior conductivity |
| Part Restoration/Creation | Copper, Nickel (Electroforming) | Repair worn parts, manufacture complex components |
Ready to engineer the perfect surface properties for your components?
At KINTEK, we specialize in providing the lab equipment and consumables essential for precise electrolytic deposition processes. Whether your goal is corrosion resistance, enhanced conductivity, or superior wear resistance, our solutions help you achieve consistent, high-quality results.
Contact our experts today to discuss how we can support your laboratory's specific coating and surface engineering needs.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- How do specialized electrolytic cells facilitate electrochemical testing? Enhance Stainless Steel Corrosion Analysis
- What are the advantages of using an undivided electrolytic cell for Acid Red-20? Boost Efficiency and Speed
- What is the primary purpose of nitrogen purging in Alloy 22 electrochemical cell testing? Ensure Data Accuracy
- Why are ion exchange membranes essential in electrochemical cell configurations? Optimize Cell Efficiency and Safety
- What are the advantages of using a three-electrode electrolytic cell system? Precise Ionic Liquid Analysis
- How should H-type electrolytic cells with glass components be handled? A Guide to Safe and Long-Lasting Use
- What are the advantages of electrochemical deposition? Unlock Precision, Cost, and Conformal Coating
- Why is a glass electrochemical cell with a plexiglass lid used for Zr2.5Nb alloys? Ensure Precision in Corrosion Tests



















