Introduction to Steady State Measurements
Electrode Reaction Processes
Electrode reactions are multifaceted processes that encompass both non-Faraday and Faraday mechanisms. These mechanisms are distinctly reflected in the respective current densities: non-Faraday and Faraday current densities.
-
Non-Faraday Processes: These processes do not involve charge transfer across the electrode-electrolyte interface. Instead, they typically involve adsorption or desorption phenomena, surface diffusion, or other surface-related interactions that do not contribute to the net charge transfer. The non-Faraday current density is indicative of these surface-bound processes, providing insights into the electrode's surface state and dynamics.
-
Faraday Processes: In contrast, Faraday processes involve the actual transfer of charge across the electrode-electrolyte interface, leading to chemical transformations such as oxidation or reduction reactions. The Faraday current density is a direct measure of these charge transfer processes, offering critical information about the kinetics and thermodynamics of the electrode reactions.

Understanding the interplay between these two types of processes is crucial for accurately interpreting steady-state measurements in electrochemistry. The balance between non-Faraday and Faraday current densities provides a comprehensive view of the electrode's behavior under various conditions, aiding in the optimization of electrochemical systems for applications ranging from energy storage to catalysis.
Steady State vs Transient Measurements
Steady state measurements in electrochemistry are characterized by the stabilization of Faraday current density, which occurs when the electrode reactions reach a state of equilibrium where the net flow of charge carriers remains constant over time. This is in stark contrast to transient measurements, where the Faraday current density is time-dependent, reflecting the dynamic nature of electrode processes as they evolve from one state to another.
In steady state conditions, the rates of the forward and reverse reactions at the electrode surface are balanced, resulting in a stable current density that does not fluctuate with time. This stability allows for precise measurements and analysis of the electrode kinetics and reaction mechanisms. On the other hand, transient measurements capture the initial stages of electrode reactions, providing insights into the time-dependent behavior of current density as the system transitions from one steady state to another.
The distinction between steady state and transient measurements is crucial for understanding the underlying mechanisms of electrode reactions. Steady state measurements are particularly useful for studying the long-term behavior and stability of electrochemical systems, while transient measurements offer a detailed view of the system's response to changes in experimental conditions, such as potential or current perturbations.
To summarize, while steady state measurements provide a snapshot of the stable conditions in an electrochemical system, transient measurements offer a dynamic perspective on how these conditions are established and altered over time. Both methods are essential for a comprehensive understanding of electrochemical processes, each contributing unique insights into the behavior of electrode reactions.
Steady State Characteristics
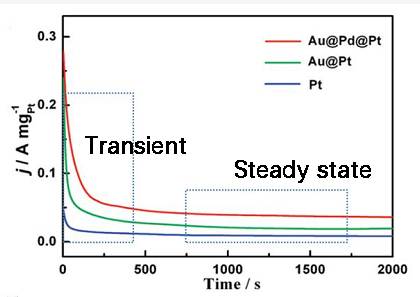
Steady State vs Equilibrium State
In the realm of electrochemistry, understanding the distinction between steady state and equilibrium state is crucial for accurate measurements and analysis. Steady state is characterized by the presence of a net current, which indicates ongoing electrochemical processes that maintain a stable condition over time. This stands in stark contrast to the equilibrium state, where no net current flows, signifying a balance between the forward and reverse reactions.
To elucidate this difference, consider the following points:
-
Net Current Presence: In a steady state, the net current is non-zero, reflecting the continuous operation of electrochemical reactions. This is essential for processes where the system needs to sustain a constant output or condition, such as in batteries or fuel cells.
-
No Net Current in Equilibrium: Conversely, an equilibrium state is marked by the absence of a net current. Here, the rates of the forward and reverse reactions are equal, resulting in no overall change in the system. This is akin to a chemical system at rest, where the concentrations of reactants and products remain constant.
| Aspect | Steady State | Equilibrium State |
|---|---|---|
| Net Current | Present | Absent |
| Reaction Dynamics | Continuous, stable reactions | Balanced forward and reverse reactions |
| System Stability | Stable over a certain period | Permanently stable, no net change |
This distinction is pivotal in various electrochemical applications, as it helps researchers and engineers determine the operational conditions that best suit their needs, whether it be maintaining a continuous flow of current or achieving a balanced, stable system.
Relative Nature of Steady State
Steady state is not an absolute concept; rather, it is a relative one. In electrochemical systems, a state is considered steady when the conditions—such as current density, potential, and concentration profiles—remain stable over a specified period. This stability is crucial for accurate measurements and reliable data analysis.
The relative nature of steady state implies that what is considered steady can vary depending on the context and the specific requirements of the experiment. For instance, in some experiments, a steady state might be reached within minutes, while in others, it could take hours or even days. The duration over which conditions are stable is often determined by the sensitivity of the measurement instruments and the rate at which the system can reach equilibrium.
Moreover, the concept of steady state is often compared to transient states, where conditions change over time. In contrast to transient measurements, steady state measurements provide a snapshot of the system when it has settled into a predictable pattern. This predictability is essential for understanding the underlying electrochemical processes and for making meaningful comparisons between different experimental conditions.
In summary, the steady state is a dynamic equilibrium where conditions are stable but not necessarily constant. It is a relative concept that depends on the specific conditions and the duration over which stability is observed. This understanding is fundamental for interpreting steady state measurements in electrochemistry accurately.
Techniques in Steady State Measurements
Constant Potential Method
The Constant Potential Method is a pivotal technique in electrochemical studies, particularly in steady state measurements. This method employs a constant potential meter to meticulously control the potential, ensuring that the potential changes are either static or dynamic. By maintaining a consistent potential, researchers can observe and analyze the behavior of electrode reactions under controlled conditions.
In static potential applications, the potential remains unchanged throughout the experiment, allowing for detailed observation of the system's response to a fixed condition. On the other hand, dynamic potential applications involve varying the potential over time, providing insights into how the system adapts to changing conditions. This dual approach enables a comprehensive understanding of both stable and evolving electrochemical processes.
The Constant Potential Method is instrumental in achieving steady state conditions, where the Faraday current density stabilizes. This is crucial for accurate measurement and interpretation of electrode kinetics, as it eliminates the complications arising from transient effects. By controlling the potential, researchers can systematically explore the relationship between potential and current density, facilitating the determination of critical reaction parameters and characteristics.
In summary, the Constant Potential Method offers a robust framework for controlling and analyzing electrochemical processes, whether under static or dynamic conditions. Its ability to stabilize potential and current density makes it an indispensable tool in the study of electrode reactions and their kinetics.
Constant Current Method
The Constant Current Method is a pivotal technique in electrochemical studies, particularly in the realm of steady state measurements. This method involves the meticulous control of an external current to ascertain stable potential values across varying current densities. By maintaining a consistent current, researchers can observe how the potential of the electrode system responds, providing valuable insights into the electrochemical behavior of the system.
One of the primary advantages of the Constant Current Method is its ability to stabilize the electrochemical system, allowing for precise measurements of potential changes. This stability is crucial for understanding the kinetics of electrode reactions, as it eliminates the transient effects that are common in time-dependent measurements. The method is particularly useful in scenarios where the current density is a critical parameter, such as in the study of corrosion, battery performance, and electrodeposition processes.
In practical applications, the Constant Current Method is often employed in conjunction with other techniques, such as the Rotating Disk Electrode (RDE) and the Steady State Polarization Curve. These combined approaches enable a comprehensive analysis of the electrode kinetics, providing a deeper understanding of the reaction mechanisms and kinetic parameters. For instance, by systematically varying the current density and measuring the corresponding potential, researchers can construct polarization curves that reveal critical points such as the corrosion potential and the limiting current density.
Moreover, the Constant Current Method is instrumental in the development of electrochemical sensors and devices. By precisely controlling the current, it is possible to optimize the performance of these devices, ensuring reliable and accurate measurements. This method is also essential in the calibration of electrochemical instruments, as it provides a standardized approach to measuring potential under controlled conditions.
In summary, the Constant Current Method plays a vital role in the field of electrochemistry, offering a robust approach to studying electrode reactions and system stability. Its ability to provide stable and reproducible measurements makes it an indispensable tool for both fundamental research and practical applications.
Steady State Polarization Curve
The steady state polarization curve stands as a pivotal technique in the realm of electrochemistry, serving as a critical tool for unraveling the intricate dynamics of electrode reactions. This method is instrumental in not only elucidating the fundamental characteristics of these reactions but also in extracting essential kinetic parameters that govern their behavior. By systematically mapping the relationship between electrode potential and current density, the polarization curve offers a comprehensive view of how different factors influence the overall reaction kinetics.
To delve deeper into the significance of the steady state polarization curve, it is essential to understand its role in the broader context of electrochemical studies. Unlike transient measurements, which focus on time-dependent changes, steady state measurements aim to stabilize the Faraday current density, thereby providing a more stable and reliable basis for analysis. This stability is crucial for accurately determining the kinetic parameters, such as reaction rate constants and transfer coefficients, which are pivotal for designing efficient electrochemical systems.
Moreover, the steady state polarization curve is particularly valuable in distinguishing between various reaction pathways and mechanisms. By analyzing the curve, researchers can identify the presence of multiple reaction steps, adsorption processes, and other interfacial phenomena that might otherwise remain obscured. This capability is further enhanced when combined with techniques like the Rotating Disk Electrode (RDE), which helps to converge electrode reactions to a steady state more rapidly, thereby minimizing the influence of transient effects.
In essence, the steady state polarization curve is not merely a diagnostic tool but a cornerstone in the systematic study of electrode kinetics. Its ability to provide detailed insights into reaction mechanisms and kinetic parameters makes it an indispensable method for both fundamental research and practical applications in electrochemistry.
Rotating Disk Electrode (RDE)
The Rotating Disk Electrode (RDE) is a specialized hydrodynamic working electrode employed in a three-electrode system, primarily designed to converge electrode reactions to a steady state. This technique is particularly effective in eliminating the effects of the double layer, which is a region of ionic charge near the electrode surface that can interfere with accurate measurements. By controlling the electrolyte flow, the RDE ensures a consistent and predictable mass transport of reactants to the electrode surface, thereby facilitating precise electrochemical studies.

In practical applications, the RDE is used in a variety of electrochemical experiments, including corrosion studies, fuel cell research, and catalyst development. The electrode's rotation during experiments creates a constant flux of analyte to the electrode, which is crucial for maintaining steady-state conditions. This setup is particularly advantageous in scenarios where defined mass transport to the sample electrode is essential, such as in catalyst evaluation.
The structure of the RDE includes a conductive disk embedded in an inert, non-conductive polymer or resin, which is then attached to an electric motor with fine control over the electrode's rotation rate. The disk, typically made from noble metals like platinum or gold, or glassy carbon, can be fabricated from any conductive material based on specific experimental requirements. This flexibility in material choice, combined with the precise control over rotation, makes the RDE a versatile tool in the field of electrochemistry.
In analytical chemistry, the RDE is utilized in three-electrode systems for hydrodynamic voltammetry. Its rotating mechanism induces a flux of analyte to the electrode, making it ideal for investigating reaction mechanisms related to redox chemistry and other chemical phenomena. For more complex studies, the rotating ring-disk electrode can be adapted, with the ring left inactive to function solely as an RDE. This adaptability further extends the RDE's utility in diverse electrochemical applications.
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
- Flat Corrosion Electrolytic Electrochemical Cell
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Related Articles
- Revolutionizing Quality Control: The Ultimate Guide to Handheld Lithium Battery Analyzers
- Ultimate Guide to Handheld Alloy Analyzers: Features, Applications, and Advantages
- Comprehensive Guide to Handheld Lithium Battery Analyzers: Features, Applications, and Maintenance
- The Architecture of Certainty: Mastering Control in Multifunctional Electrolytic Cells
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations















