It is easy to overlook the container. In electrochemistry, we obsess over the catalyst, the voltage, and the electrolyte. We calculate potentials and analyze curves. But we often treat the cell body—the glass vessel holding it all together—as an afterthought.
This is a mistake. The cell body is not just a bucket; it is the stage where the reaction performs.
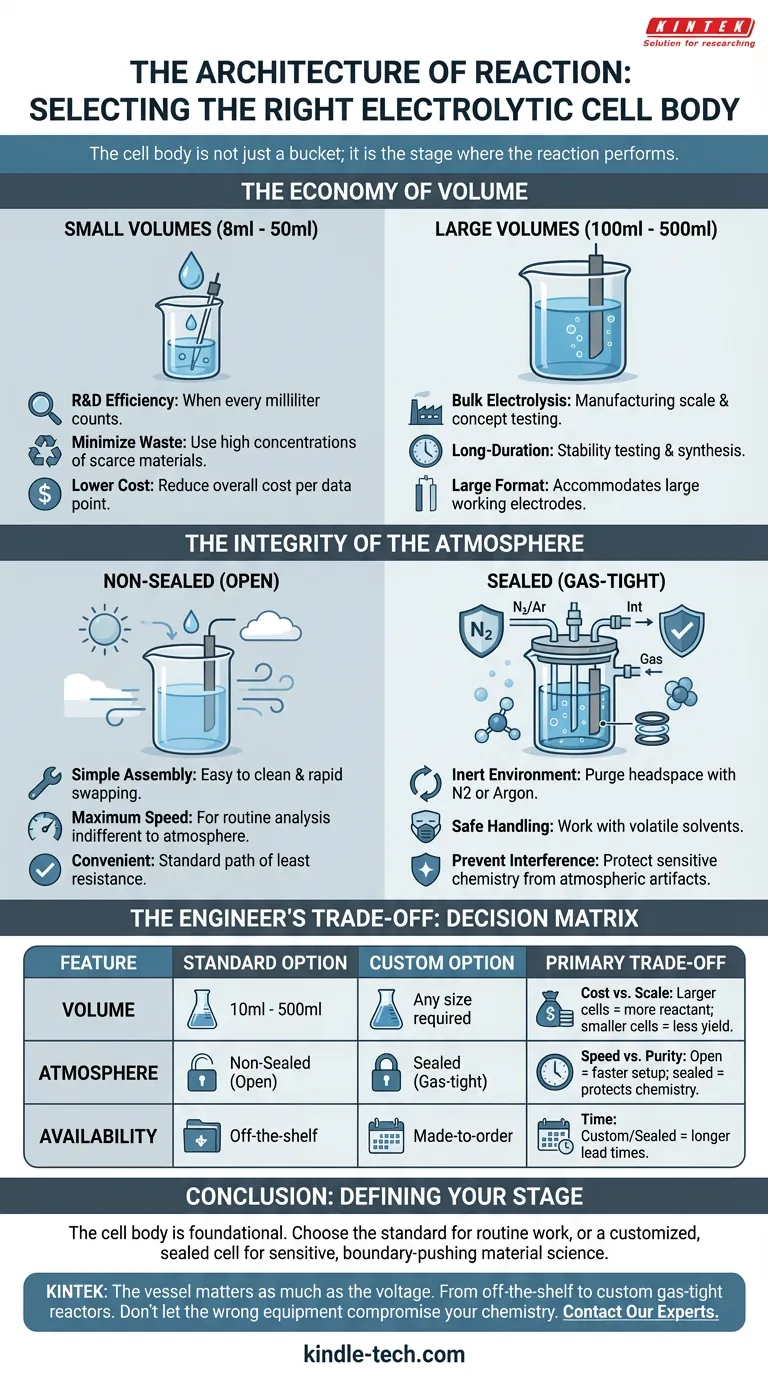
If the stage is too large, you waste precious resources. If it is open when it should be closed, the atmosphere becomes a silent contaminant. The choice of the electrolytic cell body is a fundamental engineering decision that defines the quality of your data.
The Economy of Volume
The first constraint in any experiment is scarcity.
Your choice of cell volume—ranging typically from 8ml to 500ml—is a direct reflection of the value of your materials.
The Case for Small Volumes (8ml - 50ml)
In Research and Development, efficiency is the priority. When you are synthesizing a novel catalyst or using an expensive electrolyte, every milliliter counts.
A smaller cell (commonly 8ml to 80ml for three-electrode systems) allows you to:
- Minimize waste.
- Work with high concentrations of scarce materials.
- Reduce the overall cost per data point.
The Case for Large Volumes (100ml - 500ml)
Conversely, there are times when scale is the objective. If your goal is bulk electrolysis, you are no longer just testing a concept; you are manufacturing a product.
Larger cells are engineered for:
- Long-duration stability testing.
- Synthesizing significant quantities of material.
- Accommodating large-format working electrodes.
The Integrity of the Atmosphere
Once you have determined the scale, you must address the environment. The distinction between a sealed and a non-sealed cell is not a matter of preference; it is a matter of chemical necessity.
The Open Cell (Non-Sealed)
This is the standard. It is the path of least resistance.
Non-sealed cells are simpler to assemble, easier to clean, and allow for rapid swapping of electrodes. For routine analysis where the reaction is indifferent to oxygen or humidity, the open cell offers maximum speed and convenience.
The Sanctuary (Sealed)
However, many electrochemical reactions are fragile. Oxygen is an aggressive scavenger; moisture is a disruptor.
For these sensitive systems, a sealed cell is mandatory. Through the use of O-rings, threaded caps, or ground glass joints, a sealed cell creates a gas-tight environment. This allows you to:
- Purge the headspace with inert gases like Nitrogen or Argon.
- Work safely with volatile solvents.
- Prevent atmospheric interference from skewing your current response.
The Engineer’s Trade-off
Every design choice carries a cost. The "perfect" cell does not exist in a vacuum; it exists in the context of your specific experiment.
The following table outlines the decision matrix for selecting the correct body:
| Feature | Standard Option | Custom Option | Primary Trade-off |
|---|---|---|---|
| Volume | 10ml - 500ml | Any size required | Cost vs. Scale: Larger cells require more reactant; smaller cells save money but limit yield. |
| Atmosphere | Non-Sealed (Open) | Sealed (Gas-tight) | Speed vs. Purity: Open cells are faster to set up; sealed cells protect sensitive chemistry. |
| Availability | Off-the-shelf | Made-to-order | Time: Custom or sealed configurations often require longer lead times. |
Conclusion: Defining Your Stage
The cell body is the foundational hardware of your experiment.
If you are teaching students or running routine aqueous tests, the standard 50ml open cell is a robust workhorse. But if you are pushing the boundaries of material science with air-sensitive catalysts, a customized, low-volume sealed cell is the only way to ensure your results are real, rather than artifacts of the atmosphere.
At KINTEK, we understand that the vessel matters as much as the voltage. We provide a spectrum of electrolytic cells—from standard off-the-shelf units to fully customized, gas-tight reactors—designed to fit the precise architecture of your research.
Don't let the wrong equipment compromise your chemistry. Contact Our Experts
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Super Sealed Electrolytic Electrochemical Cell
Related Articles
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell




















