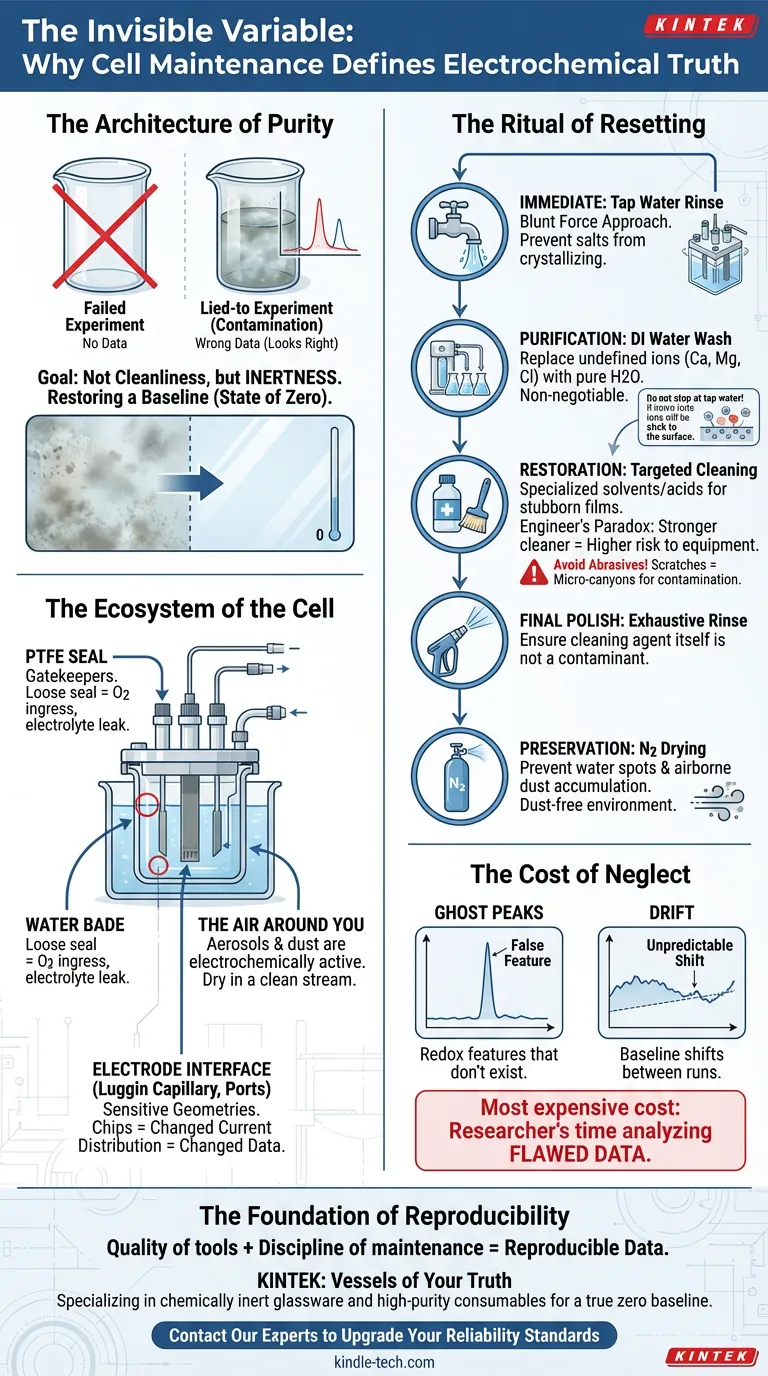
The Architecture of Purity
In the world of electrochemistry, there is a distinct difference between a failed experiment and a lied-to experiment.
A failed experiment gives you no data. A lied-to experiment gives you wrong data that looks right.
The source of that lie is almost always contamination.
When working with a five-port water bath electrolytic cell, you are dealing with a fragile, precision instrument designed to act as a blank canvas for ionic interaction. The moment that canvas has a smudge—a microscopic residue of a past reaction or a trace of cleaning detergent—the canvas becomes part of the painting.
The goal of maintenance is not cleanliness. It is inertness.
You are attempting to return the universe of your glass cell to a state of zero. This requires a shift in mindset from "washing dishes" to "restoring a baseline."
The Ritual of Resetting
The standard cleaning protocol is a defense mechanism against entropy. It follows a logical hierarchy of solvent power.
1. The Rough Draft (Tap Water) Immediately after the experiment, the clock starts. Residues begin to adhere. Disassembling the cell and rinsing with tap water removes the bulk electrolyte.
This is the blunt force approach. It clears the stage.
2. The refinement (Deionized Water) Tap water is full of ions—calcium, magnesium, chloride. If you stop at tap water, you haven't cleaned the cell; you've just coated it in new impurities.
Multiple rinses with deionized (DI) or distilled water are non-negotiable. You are replacing undefined ions with pure H2O.
3. The Deep Clean (Chemical Intervention) Sometimes, the ghost of the previous experiment refuses to leave.
You may need specialized cleaners like Alconox for organic residues. In extreme cases, chemists turn to Aqua Regia or chromic acid.
But here lies the "Engineer's Paradox": The stronger the cleaner, the higher the risk to the equipment.
Glass is chemically resistant, but it is not invincible. Physical scrubbing scratches the surface, creating micro-canyons where bacteria and ions hide. Harsh chemicals can etch the glass or ruin the optical clarity needed for photo-electrochemical work.
The Ecosystem of the Cell
An electrolytic cell does not exist in a vacuum. It relies on a system of seals and connections that are often overlooked until they fail.
The PTFE Seal Your stoppers and seals are the gatekeepers. Over time, polymers creep and deform. A loose seal allows atmospheric oxygen to seep in, ruining an anaerobic study, or allows electrolyte to leak out into the water bath.
The Electrode Interface The Luggin capillary and electrode ports are the most sensitive geometries in the cell. If these are chipped during cleaning, the current distribution changes. Your geometry changes. Your data changes.
The Air Around You If you clean your cell perfectly but dry it in a dusty room, you have wasted your time. Aerosols and dust are electrochemically active. The drying phase requires a clean nitrogen stream or a dust-free cabinet.
The Cost of Neglect
We often think of maintenance as a chore that delays "real work."
This is a psychological error.
Maintenance is the real work. The electrochemical measurement is just the final receipt.
If you neglect the cleaning protocol, two things happen:
- Ghost Peaks: You see redox features that don't exist.
- Drift: Your baseline shifts unpredictably between runs.
The most expensive thing in a laboratory is not the equipment. It is the researcher's time spent analyzing data that is fundamentally flawed.
Summary of Protocol
The following table outlines the systematic restoration of the cell's integrity.
| Phase | Action | The "Why" |
|---|---|---|
| Immediate | Tap Water Rinse | Prevent salts from crystallizing and adhering to the glass. |
| Purification | DI Water Wash | Remove the ions introduced by tap water. |
| Restoration | Targeted Cleaning | Use solvents/acids only for stubborn films. Avoid abrasives. |
| Final Polish | Exhaustive Rinse | Ensure the cleaning agent itself doesn't become the contaminant. |
| Preservation | N2 Drying | Prevent water spots and airborne dust accumulation. |
The Foundation of Reproducibility
There is a romance to the perfect experiment—where the theoretical prediction overlays perfectly with the observed data.
That alignment is built on the quality of your tools and the discipline of your maintenance.
At KINTEK, we understand that your equipment is the vessel of your truth. We specialize in providing lab equipment and consumables that respect the precision of your work. From chemically inert glassware designed to withstand rigorous cleaning cycles, to the high-purity consumables that ensure your baseline remains zero.
Don't let a dirty cell be the reason your breakthrough is delayed.
Contact Our Experts to discuss how KINTEK can upgrade your laboratory's reliability standards.
Visual Guide
Related Products
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Cylindrical Press Mold with Scale for Lab
- High Performance Laboratory Freeze Dryer
- Custom PTFE Teflon Parts Manufacturer for Acid and Alkali Resistant Chemical Powder Material Scoops
- Laboratory manual slicer
Related Articles
- Preparation of Graphene by Chemical Vapor Deposition (CVD)
- The Silent Interface: Mastery Over Electrode Decay
- All About ACTIVATED CARBON THERMAL REGENERATION
- Preparation and Transfer Technology of Graphene by Chemical Vapor Deposition
- The Invisible Architecture of Precision: Mastery Before the Current Flows