Introduction to Reference Electrodes
Definition and Function
A reference electrode serves as a crucial component in electrochemical studies, functioning as a benchmark against which the potential difference relative to a study electrode is measured. This measurement is essential for accurately assessing the behavior of the study electrode under various conditions. Typically, the potential of a reference electrode is gauged in relation to a reversible standard hydrogen electrode (RHE), which is considered the universal standard due to its well-defined and stable potential.
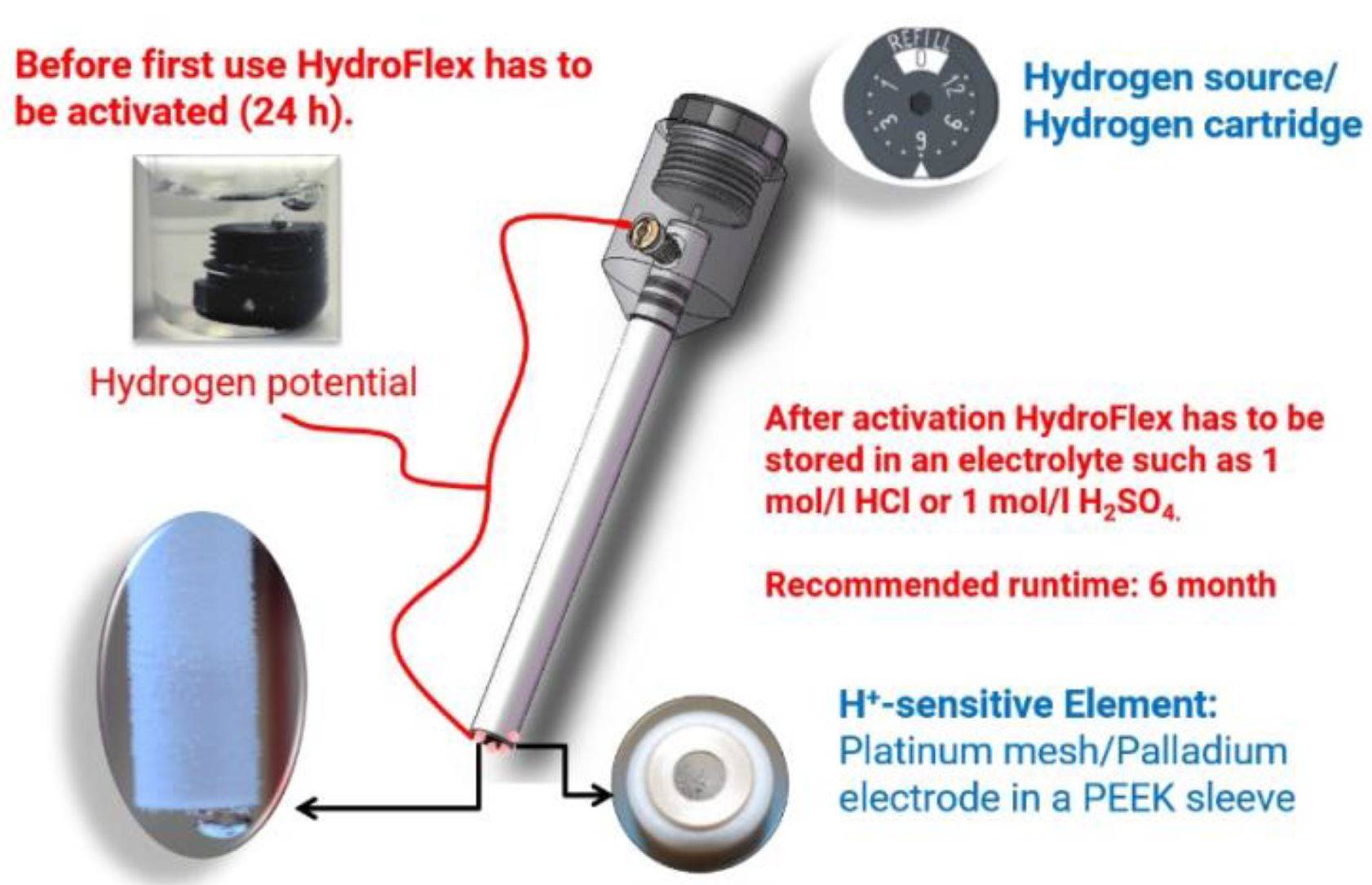
The role of the reference electrode extends beyond mere measurement; it ensures the consistency and reliability of electrochemical data. By providing a stable and known potential, it allows for precise comparisons between different experiments and conditions. This stability is crucial in fields such as corrosion studies, battery research, and environmental monitoring, where even minor variations in potential can significantly impact results.
In practical applications, the reference electrode must meet specific criteria to ensure its effectiveness. It should exhibit a known and stable potential over time and under varying conditions, ensuring that the measurements remain consistent. Additionally, the electrode must be reversible, meaning it can undergo oxidation and reduction processes without changing its fundamental properties. The electrolyte within the reference electrode should also be chemically inert, preventing any unwanted reactions with the electrolyte in the electrolytic cell or other related substances.
For instance, in a typical setup, the reference electrode might be paired with an Ag/AgCl electrode or a Hg/Hg2SO4 electrode, each with its specific electrolyte solution. These combinations ensure that the reference electrode maintains its stability and accuracy, providing reliable data for the study electrode's potential measurements.
Conditions for a Good Reference Electrode
A reference electrode must exhibit several critical characteristics to function effectively in electrochemical studies. Firstly, it must maintain a known and stable potential throughout its use. This stability ensures that the potential measurements taken are reliable and consistent, which is crucial for accurate data collection.
Secondly, the electrode should be reversible, meaning it can undergo oxidation and reduction reactions without significant changes in its potential. This reversibility is essential for maintaining the electrode's integrity and ensuring that it can be used repeatedly without degradation.
Additionally, the electrolyte within the reference electrode must be carefully chosen. It should not chemically react with the electrolyte in the electrolytic cell or any related substances. Such reactions could alter the electrode's potential, leading to erroneous measurements. For instance, if the electrolyte in the reference electrode reacts with the sample solution, it could cause a shift in the reference potential, compromising the accuracy of the readings.
Moreover, the reference electrode should be compatible with the sample being measured. This compatibility ensures that there are no unwanted chemical interactions between the sample and the electrolyte, which could affect the stability of the electrode's potential. For example, certain chemicals in the sample might degrade the material of the electrode, necessitating the selection of appropriate materials like glass, epoxy, or other suitable substances.
In practical applications, it is often more effective to use a separate sensing (half-cell) and reference electrode if the different parts of the electrode are expected to have different lifespans. This separation allows for easier replacement of worn-out components without discarding the entire electrode. Furthermore, in some specialized applications, using a separate reference electrode is not only practical but also necessary to achieve the desired accuracy and efficiency.
For instance, in cyclic voltammetry (CV), using a simple Ag wire dipped directly into the analyte solution as a reference electrode is theoretically possible but not recommended. The slow loss of Ag+ ions could interact with the analyte, and any changes in the electrolyte solution might alter the reference potential. Instead, it is best practice to isolate the reference electrode from the analyte solution using a vycor (porous glass) frit. This setup maintains electrical contact while minimizing solution mixing, thereby preserving the stability of the reference potential.
Care must also be taken to prevent the vycor frit from drying out, as this can cause the electrolyte salt to crystallize in the pores, rendering the electrode unusable. Regular checks, such as attempting to squeeze liquid through the frit using a pipette bulb, can help ensure its integrity. Commercially available aqueous Ag/AgCl reference electrodes should be stored in the dark and submerged in solutions identical to the solution inside the reference electrode, typically saturated KCl. Over time, Ag/AgCl electrodes may develop a white buildup on the wire and drift from their advertised reference potential, necessitating careful monitoring and replacement when necessary.
In summary, a good reference electrode must combine stability, reversibility, and chemical compatibility to provide reliable and accurate potential measurements in electrochemical experiments.
Calibration and Correction of Reference Electrodes

Calibration Process
The calibration of a reference electrode is a meticulous process that ensures the accuracy and reliability of potential measurements in electrochemical studies. The procedure typically involves setting up a three-electrode system, where the reference electrode under calibration serves as the working electrode. The system is completed by incorporating an Ag/AgCl electrode as the reference electrode and a platinum (Pt) electrode as the counter electrode.
To achieve a precise calibration, the open-circuit potential monitoring test method is employed. This method involves monitoring the potential of the working electrode over time until a stable reading is obtained. The stability of the potential curve is crucial, as it indicates that the reference electrode is functioning correctly and consistently.
| Component | Role in Calibration |
|---|---|
| Working Electrode | Reference electrode under test |
| Reference Electrode | Ag/AgCl electrode |
| Counter Electrode | Pt electrode |
The open-circuit potential monitoring test method is particularly advantageous because it allows for the identification of any drift or instability in the reference electrode's potential. This method is non-invasive and does not require any external current, making it an ideal choice for maintaining the integrity of the reference electrode's environment during calibration.
In summary, the calibration process of a reference electrode is a critical step in ensuring the accuracy of electrochemical measurements. By utilizing a three-electrode system and employing the open-circuit potential monitoring test method, researchers can obtain a stable and reliable potential curve, thereby validating the performance of the reference electrode.
Correction Formula
The actual potential of the reference electrode can be accurately determined using a specific correction formula. This formula, expressed as ( E_X = x - 0.197 ), is essential for ensuring the precision and reliability of electrochemical measurements. In this equation, ( x ) represents the measured potential of the reference electrode, while the constant 0.197 corresponds to the known electrode potential of the Ag/AgCl electrode.
To understand the significance of this correction, it's important to recognize that the Ag/AgCl electrode serves as a standard reference in many electrochemical experiments. Its stable and well-documented potential allows for consistent calibration across different setups. By subtracting the Ag/AgCl electrode potential from the measured value, researchers can obtain the true potential of their reference electrode, thereby eliminating potential discrepancies and enhancing the accuracy of their data.
This correction process is particularly crucial in experiments where even minor variations in electrode potential can significantly impact results. Therefore, the formula ( E_X = x - 0.197 ) is not just a mathematical adjustment but a critical step in maintaining the integrity of electrochemical measurements.
Commonly Used Reference Electrodes
Hg/Hg₂SO₄ Electrode
The Hg/Hg₂SO₄ electrode is constructed with a solid amalgamation of mercury and mercury(I) sulfate, encapsulated within a solution of sulfate ions. Specifically, the electrode configuration is represented as Hg/Hg₂SO₄(solid)/SO₄²⁻, and it is typically immersed in a 0.1M sulfate solution. This setup ensures a stable and well-defined potential, making it a reliable reference in various electrochemical applications.
Unlike other reference electrodes, such as the Silver-Silver Chloride electrode, which relies on the precipitation of AgCl in the electrolyte, the Hg/Hg₂SO₄ electrode leverages the solid-state interaction between mercury and its sulfate compound. This unique structure not only provides a robust foundation for potential measurement but also minimizes the risk of contamination or instability, which can be common in electrodes that involve liquid-liquid interfaces.
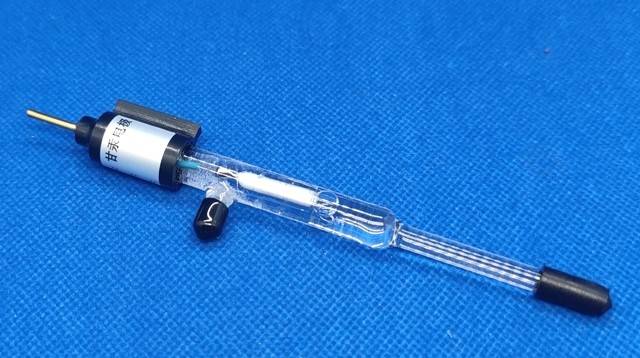
The use of a 0.1M sulfate solution further enhances the electrode's performance by maintaining a consistent ionic environment around the mercury amalgam. This concentration is carefully chosen to balance the need for sufficient ion mobility with the requirement for minimal interference from the electrolyte. As a result, the Hg/Hg₂SO₄ electrode remains a popular choice for applications where high precision and long-term stability are paramount.
Saturated Mercuric Acid Electrode
The Saturated Mercuric Acid Electrode (SMAE) is a critical component in electrochemical studies, particularly known for its structure comprising mercury (Hg), mercurous chloride (Hg₂Cl₂, also known as calomel), and a saturated potassium chloride (KCl) solution. This electrode is designed to maintain a stable and reproducible potential, which is essential for accurate measurements in various electrochemical experiments.
The SMAE's structure is straightforward yet highly effective. It consists of a mercury pool at the bottom, on which a layer of solid calomel is deposited. This calomel layer acts as an intermediary between the mercury and the electrolyte solution. The built-in saturated KCl solution ensures that the electrode remains in a state of equilibrium, providing a consistent reference potential.
One of the key advantages of the SMAE is its ability to operate in a saturated KCl environment, which helps in maintaining a high concentration of chloride ions. This saturation is crucial as it minimizes potential fluctuations and ensures a stable reference potential over extended periods, making it particularly suitable for long-term experiments.
In summary, the SMAE's design, with its Hg/Hg₂Cl₂(solid)/KCl structure and the use of a saturated KCl solution, offers a robust and reliable reference potential, making it a preferred choice in many electrochemical applications.
Silver/Silver Chloride Electrode
The Silver/Silver Chloride (Ag/AgCl) electrode is a widely used reference electrode due to its cost-effectiveness and reduced toxicity compared to other reference electrodes like the Calomel electrode. This electrode is composed of a silver wire (Ag) coated with a layer of solid silver chloride (AgCl), which is then immersed in a solution saturated with both potassium chloride (KCl) and silver chloride (AgCl). The structure of the electrode can be represented as Ag/AgCl(solid)/KCl.
The electrode operates based on the following half-reaction:
$$ \text{AgCl(s)} + \text{e}^- \leftrightarrow \text{Ag(s)} + \text{Cl}^-(\text{sat'd}) $$
At 25°C, this reaction yields a potential of 0.197 V with respect to the Standard Hydrogen Electrode (SHE). This value slightly deviates from the standard electrode potential (E0) of 0.222 V due to the contribution of both KCl and AgCl to the chloride activity, which is not exactly unity.
The Ag/AgCl electrode is favored for several reasons:
- Stability: It maintains a stable half-cell potential over time.
- Temperature Dependence: The potential changes by approximately 0.5 – 1.0 mV/°C, which is relatively minimal.
- Saturated Solution: The loss of electrolyte to evaporation does not alter the saturated nature of the solution, thereby preserving the electrode's potential.
A schematic representation of the Ag/AgCl reference electrode is often illustrated to provide a clear visual understanding of its construction and operation.
Mercury/Mercury Oxide Electrode
The Mercury/Mercury Oxide Electrode (MMO Electrode) is a critical component in electrochemical studies, characterized by its unique structure. This electrode is composed of a mercury (Hg) metal layer in direct contact with a solid layer of mercury oxide (HgO), all immersed in a concentrated potassium hydroxide (KOH) solution. Specifically, the built-in solution is typically a 1M KOH solution, which plays a vital role in maintaining the electrode's stability and performance.
The MMO Electrode is particularly valued for its ability to provide a stable and reproducible potential, making it an ideal choice for various electrochemical applications. The KOH solution not only ensures the electrode's functionality but also helps in preventing contamination and maintaining the electrode's longevity. This setup allows the MMO Electrode to serve effectively as a reference electrode, offering consistent potential readings that are crucial for accurate electrochemical measurements.
In summary, the Mercury/Mercury Oxide Electrode stands out due to its robust structure and the use of a 1M KOH solution, which collectively contribute to its reliability and effectiveness in electrochemical research.
Selection and Maintenance of Reference Electrodes
Selection Criteria
When selecting a reference electrode, it is crucial to ensure that the built-in solution matches the research system to prevent any potential contamination. This matching is essential because the electrolyte of the reference electrode should not react with the electrolyte in the electrolytic cell or related substances, which could compromise the accuracy of your measurements.
For instance, when working with H₂SO₄ solutions, the Hg/Hg₂SO₄ electrode is the ideal choice. This electrode, with its structure of Hg/Hg₂SO₄(solid)/SO₄²⁻, comes equipped with a built-in solution of 0.1M sulfate solution, making it perfectly suited for sulfuric acid environments. On the other hand, for chloride solutions, the Ag/AgCl electrode is the preferred option. This electrode, structured as Ag/AgCl(solid)/KCl, includes a built-in solution of 0.1M KCl solution, which is optimal for chloride-based research systems.
| Solution Type | Recommended Electrode | Electrode Structure | Built-in Solution |
|---|---|---|---|
| H₂SO₄ Solutions | Hg/Hg₂SO₄ Electrode | Hg/Hg₂SO₄(solid)/SO₄²⁻ | 0.1M Sulfate Solution |
| Chloride Solutions | Ag/AgCl Electrode | Ag/AgCl(solid)/KCl | 0.1M KCl Solution |
By carefully matching the reference electrode to the solution type, you can significantly reduce the risk of contamination and ensure more reliable and accurate electrochemical measurements.
Maintenance Tips
Proper maintenance of reference electrodes is crucial for maintaining their accuracy and longevity. To ensure optimal performance, follow these essential maintenance tips:
-
Storage Conditions: Store the reference electrode at room temperature to prevent potential fluctuations caused by extreme temperatures. Additionally, shield the electrode from direct light to avoid any degradation of the materials.
-
Filling Solution Management: Regularly replace the filling solution to maintain the electrode's potential stability. The frequency of replacement depends on the usage rate, but it is generally advisable to check and replace the solution every few weeks to ensure consistency.
-
Air Bubble Prevention: Ensure that there are no air bubbles in the built-in solution. Air bubbles can interfere with the electrode's performance by creating a barrier that disrupts the electrical connection. Regularly inspect the solution and gently tap the electrode to remove any trapped air.
By adhering to these maintenance practices, you can significantly enhance the reliability and lifespan of your reference electrodes, ensuring accurate and consistent results in your electrochemical studies.
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
- Gold Disc Electrode
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrode Polishing Material for Electrochemical Experiments
Related Articles
- Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
- Electrolytes and Electrochemical Electrodes
- A Beginner's Guide to Understanding Reference Electrodes in Electrochemistry
- Electrochemical Electrodes in Chemical Analysis
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations















