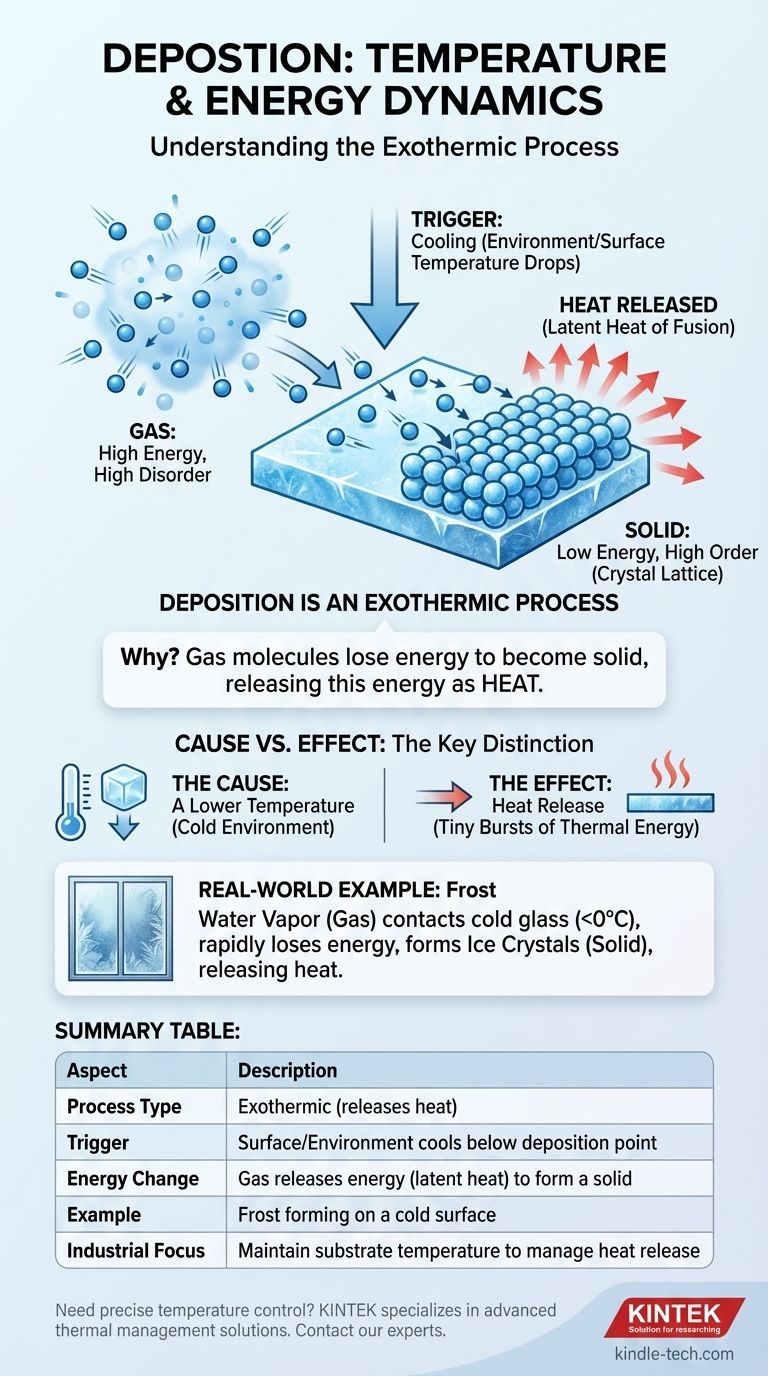
In the phase transition of deposition, energy is released as heat, making it an exothermic process. However, for deposition to occur in the first place, the temperature of a surface or the surrounding environment must drop below the substance's deposition point, which is the temperature at which it turns from a gas directly into a solid.
Deposition happens because of cooling, but the process itself releases heat. The substance must shed energy to transition from a high-energy gas to a low-energy solid, and this shed energy is released as heat into the environment.

The Core Principle: Energy and States of Matter
To understand the temperature dynamics of deposition, you must first grasp the energy levels of different states of matter. The transition between these states is entirely about managing energy.
Gas: High Energy, High Disorder
Gas particles possess high kinetic energy. They move rapidly and randomly, remaining far apart from one another with weak intermolecular forces.
Solid: Low Energy, High Order
In a solid, particles are locked into a fixed, ordered structure called a crystal lattice. They have much lower kinetic energy, mostly vibrating in place, and are held together by strong intermolecular bonds.
The Energy Bridge: Deposition
Deposition is the process of crossing the bridge from a high-energy gas to a low-energy solid. For a particle to make this transition, it must get rid of its excess kinetic energy.
Deposition as an Exothermic Process
The release of energy defines the thermal nature of deposition. It is fundamentally an exothermic process.
Why Deposition Releases Heat
When gas molecules settle onto a surface and form bonds to create a solid lattice, they move to a more stable, lower-energy state. The difference in energy between the chaotic gas phase and the ordered solid phase must be released. This released energy is known as the latent heat of fusion and is given off to the surroundings.
A Real-World Example: Frost
The formation of frost on a cold window is a perfect example of deposition. Water vapor (a gas in the air) comes into contact with a glass pane that is below freezing (0°C or 32°F). The cold glass forces the water vapor molecules to rapidly lose energy, causing them to change directly into ice crystals (a solid) without ever becoming liquid water.
Understanding the Key Distinction: Cause vs. Effect
The core of the confusion around this topic is failing to distinguish between the condition required for deposition and the result of the process itself.
The Cause: A Lower Temperature
Deposition does not happen spontaneously. It is triggered by cooling. A substance will only deposit onto a surface or within an environment that is at or below its deposition temperature. This cold environment acts as an energy sink, drawing heat away from the gas molecules.
The Effect: Heat Release
As the gas molecules lose their energy and lock into a solid structure, that energy is released as heat. If you could measure it precisely, the formation of an ice crystal on a surface releases a tiny burst of thermal energy.
The Net Result
For deposition to continue, the surrounding environment must be efficient at absorbing the latent heat being released. If the released heat were to warm the surface back up above the deposition point, the process would stop or even reverse into sublimation (solid to gas).
Making the Right Choice for Your Goal
Understanding this principle clarifies how to think about and control phase transitions.
- If your primary focus is a science exam: Remember that deposition is an exothermic process where heat is released, making it the direct opposite of sublimation, which is endothermic.
- If your primary focus is an industrial application like physical vapor deposition (PVD): Your key variable is maintaining the substrate at a sufficiently low temperature to both initiate deposition and continuously draw away the latent heat being released by the coating material.
- If your primary focus is a simple mental model: Think of frost forming on a car windshield overnight. The windshield must first get cold (the cause), and the process of frost actually forming on its surface releases a small amount of heat (the effect).
By separating the trigger (cooling) from the process (heat release), you can accurately describe the thermodynamics of any phase change.
Summary Table:
| Aspect | Description |
|---|---|
| Process Type | Exothermic (releases heat) |
| Trigger | Surface/Environment cools below deposition point |
| Energy Change | Gas releases energy (latent heat) to form a solid |
| Example | Frost forming on a cold surface |
| Industrial Focus | Maintain substrate temperature to manage heat release |
Need precise temperature control for your deposition processes? KINTEK specializes in advanced lab equipment for thermal management and phase change applications. Our solutions help you maintain the critical substrate temperatures required for efficient and consistent deposition. Contact our experts today to optimize your laboratory's capabilities!
Visual Guide
Related Products
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What is the apparatus of chemical vapor deposition? The Essential Components for Thin Film Deposition
- Are CVD diamonds worth it? Unlock Brilliant Value & Ethical Clarity
- What is the difference between physical deposition and chemical deposition? Choose the Right Thin-Film Technology
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- What is the CVD method for synthetic diamonds? Grow Lab Diamonds from Gas with Precision



















