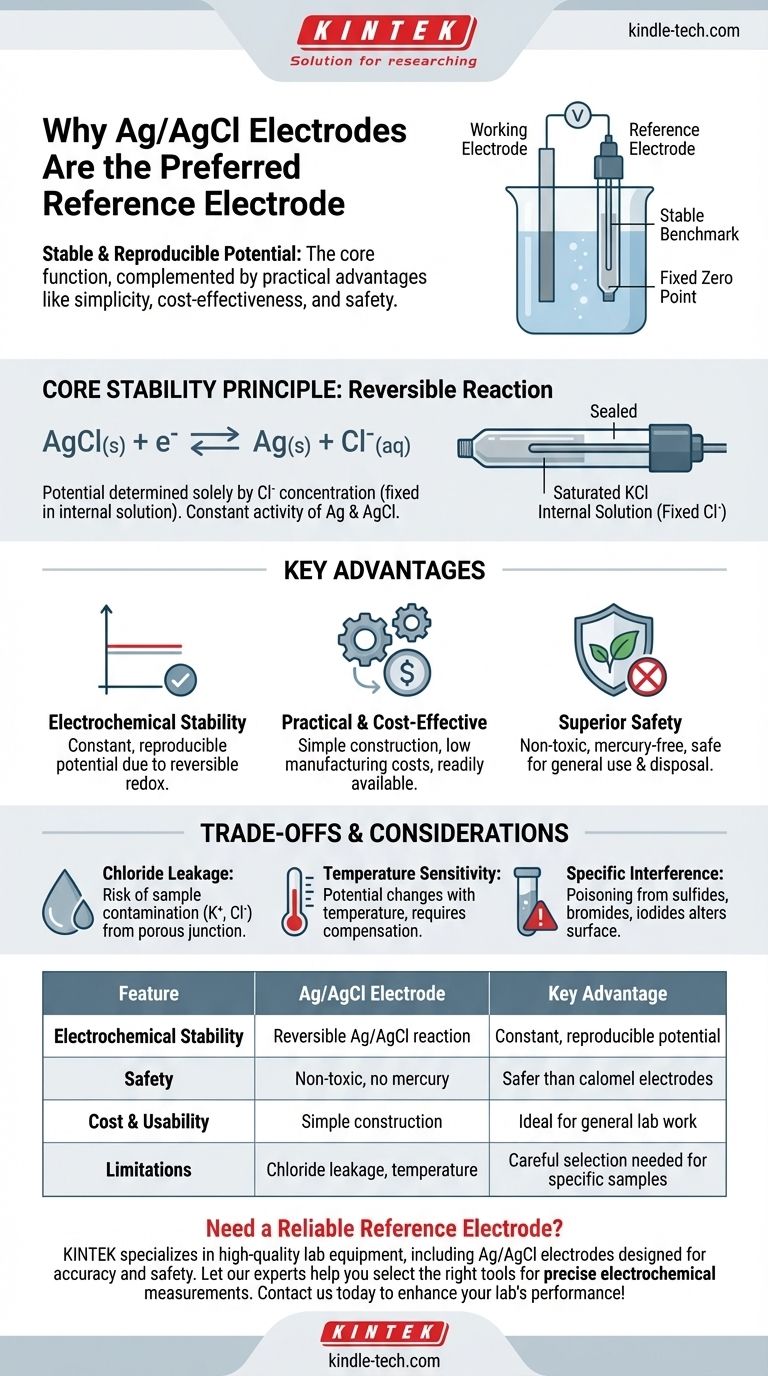
In short, the silver/silver chloride (Ag/AgCl) electrode is used as a reference electrode because it provides a highly stable and reproducible electrical potential. This core function is complemented by its practical advantages, including being simple to construct, inexpensive, and significantly less toxic than older alternatives like the calomel electrode.
A reference electrode’s primary job is to be a stable, unchanging benchmark. The Ag/AgCl electrode excels at this electrochemical task while also being safe, affordable, and easy to use, making it the dominant choice in modern electrochemistry.
Why Stability is the Cornerstone of a Reference Electrode
The Core Requirement: A Constant Potential
A reference electrode acts as a fixed "zero point" in an electrochemical cell. All other potential changes from the working electrode are measured against this stable baseline.
For a measurement to be accurate, the reference electrode's potential must not drift or change during the experiment. It must be insensitive to the composition of the sample solution it is measuring.
The Principle of a Reversible Reaction
This stability is achieved through a well-defined and reversible electrochemical reaction. In the Ag/AgCl electrode, the potential is determined solely by the concentration of chloride ions (Cl⁻) in its internal filling solution.
Because this internal solution is sealed and has a fixed concentration (typically saturated potassium chloride), the resulting electrode potential is exceptionally constant and reproducible.
How the Ag/AgCl Electrode Delivers on Key Criteria
Electrochemical Stability
The Ag/AgCl electrode is based on a simple, fast, and reversible redox reaction:
AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻(aq)
Since the activities of the solid silver (Ag) and silver chloride (AgCl) are constant, the potential depends only on the activity of the chloride ion, which is held constant within the electrode body.
Practical Advantages Over Alternatives
The widespread adoption of the Ag/AgCl electrode is also due to its significant practical benefits. It is simple to manufacture, leading to low costs for commercial electrodes.
Superior Safety Profile
Crucially, it is non-toxic. This gives it a major advantage over the once-common Saturated Calomel Electrode (SCE), which contains highly toxic mercury and poses disposal and safety hazards. For this reason, Ag/AgCl has largely replaced calomel electrodes in general laboratory use.
Understanding the Trade-offs and Limitations
No electrode is perfect for every situation. Understanding the limitations of the Ag/AgCl electrode is key to ensuring accurate measurements.
Contamination from Chloride Leakage
The most common issue is the slow leakage of the KCl filling solution from the electrode's porous junction into the sample. This can contaminate samples that are sensitive to either potassium (K⁺) or chloride (Cl⁻) ions.
For instance, measuring chloride in a water sample with a standard Ag/AgCl electrode would produce artificially high results due to this leakage.
Temperature Sensitivity
The potential of an Ag/AgCl electrode is dependent on temperature. While this relationship is predictable, it means that for high-precision work, the temperature must be controlled or its effect must be compensated for in calculations.
Interference in Specific Samples
The electrode can be "poisoned" if the sample contains substances that react with silver, such as proteins with sulfide groups, bromide ions, or iodide ions. This reaction alters the surface of the electrode and causes the potential to drift unpredictably.
Making the Right Choice for Your Goal
When selecting a reference electrode, your primary goal dictates the best choice.
- If your primary focus is general-purpose lab work (e.g., pH measurements): The standard Ag/AgCl electrode is the ideal default due to its excellent balance of stability, low cost, and safety.
- If your primary focus is analyzing samples sensitive to chloride or potassium: You must use a double-junction reference electrode, which has a second outer chamber to prevent the KCl solution from contaminating your sample.
- If your primary focus is working in non-aqueous solvents or at high temperatures: The performance of a standard Ag/AgCl can be compromised, and you may need to investigate specialized reference electrodes designed for these specific conditions.
Understanding these core principles empowers you to select not just an electrode, but the correct tool for ensuring accurate and reliable measurements.
Summary Table:
| Feature | Ag/AgCl Electrode | Key Advantage |
|---|---|---|
| Electrochemical Stability | Based on a reversible Ag/AgCl reaction | Provides a constant, reproducible potential |
| Safety | Non-toxic, no mercury | Safer than calomel electrodes |
| Cost & Usability | Simple to construct and inexpensive | Ideal for general lab work |
| Limitations | Chloride leakage risk, temperature sensitivity | Requires careful selection for specific samples |
Need a reliable reference electrode for your lab? KINTEK specializes in high-quality lab equipment and consumables, including Ag/AgCl electrodes designed for accuracy and safety. Let our experts help you select the right tools for precise electrochemical measurements. Contact us today to enhance your lab's performance!
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the typical shape and size of a metal disk electrode? A Guide to Standard and Custom Dimensions
- What methods can be used to verify the performance of a metal disk electrode? Ensure Accurate Electrochemical Results
- What is the expected lifespan of a metal disk electrode? Extend Its Life with Proper Care
- What is the purpose of selecting polycrystalline disc electrodes? Achieve Precision in Noble Metal Corrosion Research



















