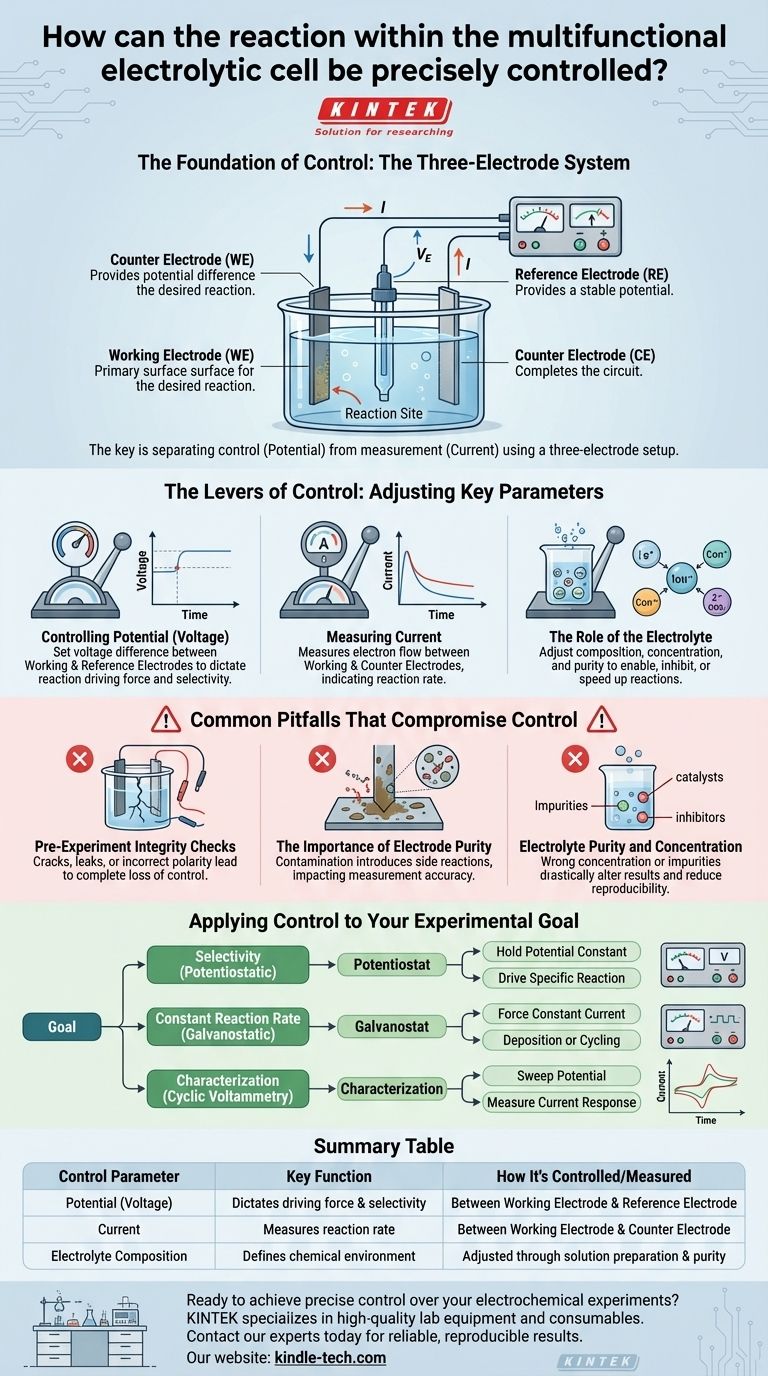
To precisely control the reaction in a multifunctional electrolytic cell, you must directly manipulate three primary parameters: the applied potential (voltage), the resulting current, and the chemical composition of the electrolyte. This control is not achieved by guesswork but through a specific hardware configuration known as the three-electrode system.
The key to precise control is not just adjusting voltage and current, but understanding that the three-electrode system is specifically designed to separate these two factors. This allows you to independently control the reaction's driving force (potential) while accurately measuring its rate (current).

The Foundation of Control: The Three-Electrode System
A multifunctional electrolytic cell's precision comes from its three-electrode design. A two-electrode system conflates variables, but a three-electrode system isolates them, enabling truly accurate control and measurement.
The Working Electrode (WE)
This is the most important component. The working electrode is the surface where the specific electrochemical reaction you want to study or control takes place. All measurements are relative to this electrode.
The Counter Electrode (CE)
The counter electrode (also called the auxiliary electrode) serves one primary purpose: to complete the electrical circuit. Current flows between the working electrode and the counter electrode. Its use ensures that the sensitive reference electrode does not have to pass significant current, which would destabilize its potential.
The Reference Electrode (RE)
This is the cornerstone of precision. The reference electrode provides a stable, known electrochemical potential. A device called a potentiostat measures and controls the voltage difference between the working electrode and this stable reference, ensuring the driving force of your reaction is exactly what you set it to be, regardless of changes occurring elsewhere in the cell.
The Levers of Control: Adjusting Key Parameters
With the three-electrode system in place, you can now use your control levers with confidence.
Controlling Potential (Voltage)
The potential you set on your potentiostat is the voltage difference applied between the working electrode and the reference electrode. This potential dictates the thermodynamic driving force for the reaction. By precisely setting this value, you can selectively target a specific chemical reaction while avoiding others that occur at different potentials.
Measuring Current
The current is the flow of electrons, which directly corresponds to the rate of your reaction. This current flows between the working electrode and the counter electrode. By controlling the potential (the cause), you can accurately measure the resulting current (the effect), giving you quantitative data on your reaction's speed.
The Role of the Electrolyte
The electrolyte is the chemical environment. Its composition, concentration, and purity determine which reactions are possible and how efficiently they can occur. Adjusting the electrolyte is like changing the rules of the game; it can enable, inhibit, or alter the speed of electrochemical processes.
Common Pitfalls That Compromise Control
Theoretical precision is meaningless without practical diligence. Failure to prepare the physical system properly is the most common source of error.
Pre-Experiment Integrity Checks
Before any experiment, confirm the electrolytic cell has no cracks or leaks. Ensure all electrical connections are secure and that the polarity is correct. A physical failure leads to a complete loss of control.
The Importance of Electrode Purity
The surface of your working electrode must be impeccably clean. Any contamination introduces unwanted side reactions, making it impossible to isolate the process you intend to study. The measured current will be a mix of your desired reaction and these unknown interferences.
Electrolyte Purity and Concentration
Using an electrolyte with the wrong concentration or unknown impurities can drastically alter the results. Impurities can act as catalysts or inhibitors, or even react themselves, invalidating your experiment and making your results impossible to reproduce.
Applying Control to Your Experimental Goal
Your control strategy depends entirely on what you want to achieve.
- If your primary focus is selectivity (a potentiostatic experiment): Your goal is to hold the potential constant between the working and reference electrodes to drive a specific reaction without initiating others.
- If your primary focus is a constant reaction rate (a galvanostatic experiment): Your goal is to force a constant current between the working and counter electrodes, which is essential for processes like controlled deposition or battery cycling.
- If your primary focus is characterization (e.g., cyclic voltammetry): Your goal is to systematically sweep the potential between the working and reference electrodes and measure the resulting current to understand the electrochemical behavior of your system.
Mastering these principles of separation and preparation is the key to achieving reliable and reproducible results in your work.
Summary Table:
| Control Parameter | Key Function | How It's Controlled/Measured |
|---|---|---|
| Potential (Voltage) | Dictates the reaction's driving force and selectivity. | Controlled between the Working Electrode and Reference Electrode. |
| Current | Measures the rate of the reaction. | Flows between the Working Electrode and Counter Electrode. |
| Electrolyte Composition | Defines the chemical environment and possible reactions. | Adjusted through solution preparation and purity control. |
Ready to achieve precise control over your electrochemical experiments?
KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. Whether you are setting up a new three-electrode system or optimizing an existing one, our expertise and reliable products ensure you get accurate, reproducible results.
Contact our experts today to discuss how we can support your research with the right equipment and consumables.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity



















