In short, sintering increases density by using thermal energy to fuse individual material particles together, systematically eliminating the empty spaces, or pores, between them. This process occurs below the material's melting point, relying on atomic diffusion to transform a loose powder compact into a solid, dense mass.
Sintering is not simply a process of compression. It is a carefully controlled thermodynamic process where atoms migrate to reduce a material's overall surface energy, causing particles to bond and pores to shrink, thereby increasing the material's final density and strength.

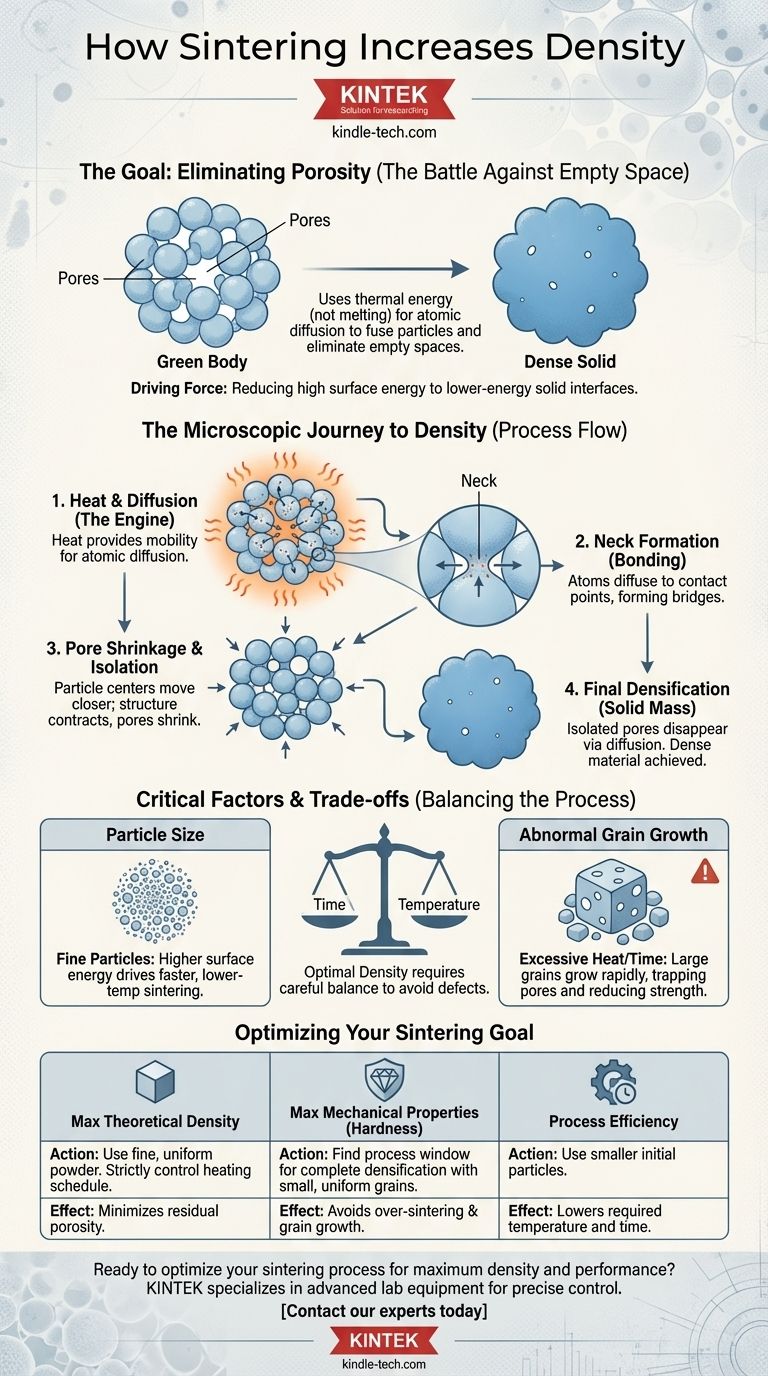
The Fundamental Goal: Eliminating Porosity
Sintering is fundamentally a battle against empty space. The process starts with a collection of individual particles and ends with a solid object, with the primary transformation being the removal of the air gaps between those initial particles.
The "Green Body" Starting Point
The process begins with a "green body," which is a loosely compacted mass of powder. This initial form has significant porosity—a network of interconnected empty spaces between the particles. Its density is far lower than that of the solid material.
Heat as the Engine for Change
Heat provides the critical energy for sintering, but its purpose is not to melt the material. Instead, it elevates the temperature enough to give the atoms within the particles mobility. This allows them to move and rearrange themselves, a process known as solid-state diffusion.
The Driving Force: Reducing Surface Energy
At the microscopic level, every particle surface represents a high-energy state compared to the material's interior. The system naturally seeks to minimize this high surface energy.
Sintering achieves this by replacing high-energy solid-to-gas interfaces (the surfaces of the pores) with lower-energy solid-to-solid interfaces, known as grain boundaries. This reduction in total energy is the fundamental driving force behind the entire densification process.
The Microscopic Mechanisms of Material Transport
As atoms gain mobility from heat, they begin to move in predictable ways that cause the material to consolidate and densify.
Neck Formation and Growth
The first stage of sintering occurs at the contact points between adjacent particles. Atoms diffuse to these points, forming small bridges or "necks." As more atoms migrate to these areas, the necks grow wider.
Particle Centers Move Closer
This neck growth effectively pulls the centers of the particles closer together. As millions of particles do this simultaneously, the entire structure contracts, and the pores between them begin to shrink.
Pore Isolation and Elimination
As the process continues, the network of pores breaks down, becoming a series of isolated, spherical voids. In the final stage, atoms continue to diffuse from the surrounding grain boundaries into these voids, causing them to shrink and, in ideal conditions, disappear entirely, resulting in a fully dense material.
Understanding the Trade-offs and Process Control
Achieving maximum density requires careful control, as several factors can either help or hinder the process. Pushing the parameters too far can be counterproductive.
The Critical Role of Particle Size
The driving force for sintering is much stronger in fine-grained materials. Smaller particles have a much higher surface-area-to-volume ratio, meaning they have more excess surface energy to be eliminated. This is why ceramic and metal powder technologies rely on very fine starting powders—it allows for more effective sintering at lower temperatures and in shorter times.
The Danger of Abnormal Grain Growth
While time and temperature are necessary for densification, too much of either can be detrimental. If the material is held at a high temperature for too long, a phenomenon called abnormal grain growth can occur.
Here, a few grains grow exceptionally large by consuming their smaller neighbors. This rapid growth can trap pores inside the new, larger grains, where they become nearly impossible to remove. This process can actually decrease the final hardness and strength of the material, creating new defects.
Balancing Time and Temperature
Achieving optimal density is a careful balance.
- Too little time or temperature: The material will be left with significant residual porosity.
- Too much time or temperature: Abnormal grain growth can trap pores and create new defects, reducing mechanical properties.
Optimizing Sintering for Your Goal
The right approach to sintering depends entirely on the desired properties of the final component. By understanding the core principles, you can tailor the process to your specific objective.
- If your primary focus is achieving maximum theoretical density: You must use fine, uniform starting powders and carefully control the heating schedule to close pores without initiating abnormal grain growth.
- If your primary focus is maximizing mechanical properties like hardness: You need to find the process window where densification is nearly complete, but the grain size remains small and uniform, as over-sintering will degrade these properties.
- If your primary focus is process efficiency: Using smaller initial particles is key, as their higher surface energy can lower the required sintering temperature and time, saving energy and increasing throughput.
Mastering these principles gives you direct control over the microstructure and, therefore, the final performance of your material.
Summary Table:
| Sintering Stage | Key Action | Effect on Density |
|---|---|---|
| Neck Formation | Atoms diffuse to particle contact points | Initial bonding begins |
| Particle Coalescence | Particle centers move closer together | Porosity decreases, density increases |
| Pore Elimination | Isolated pores shrink via atomic diffusion | Achieves near-theoretical density |
Ready to optimize your sintering process for maximum density and performance? KINTEK specializes in advanced lab equipment and consumables for materials science. Whether you're working with fine metal powders or ceramics, our solutions help you achieve precise temperature control and avoid defects like abnormal grain growth. Contact our experts today to discuss how we can support your laboratory's sintering and densification needs.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is the effect of zirconia sintering temperature? Master the Key to Strength and Stability
- What makes zirconia translucent? The Science Behind Modern Dental Aesthetics
- What is one of the newest applications for dental ceramics? Monolithic Zirconia for Full-Arch Bridges
- What is the sintering temperature of zirconium? A Guide to the 1400°C-1600°C Range for Dental Labs



















