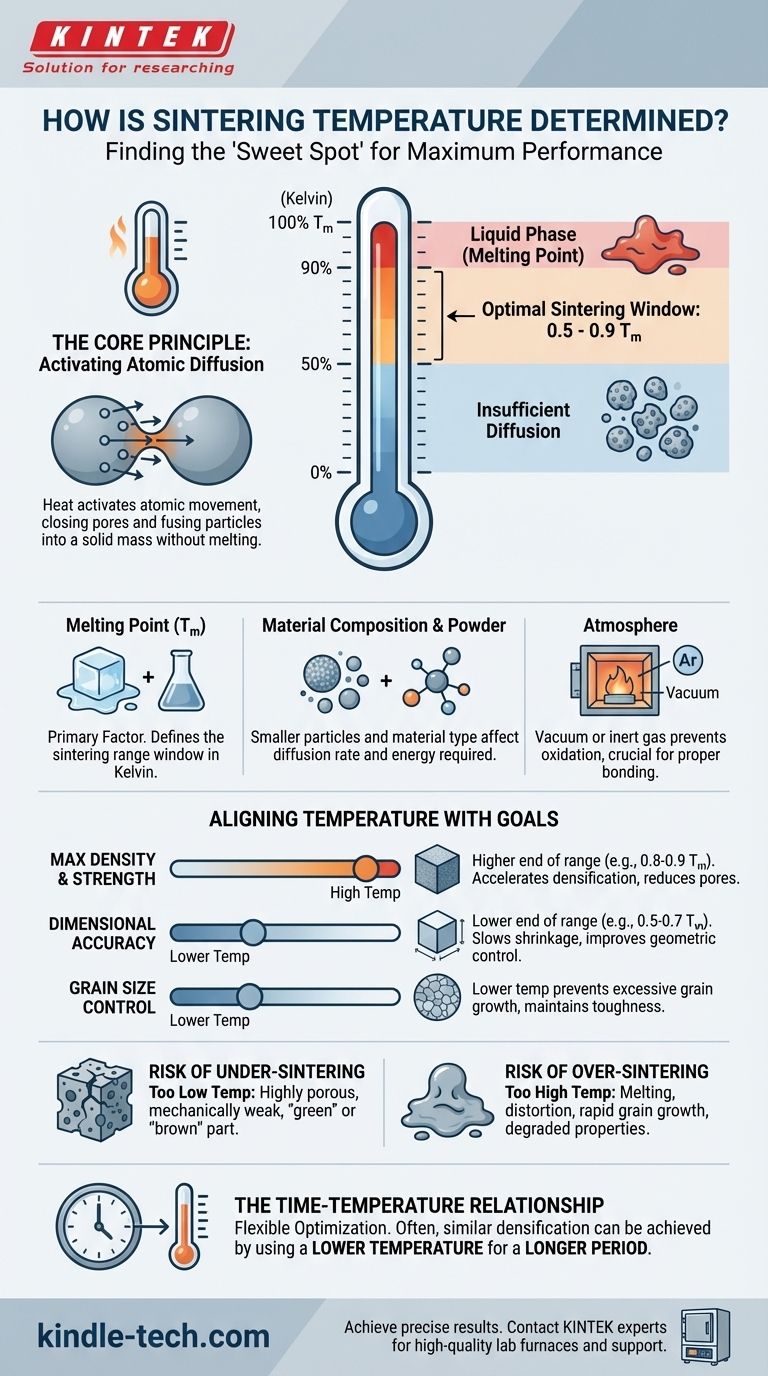
In short, sintering temperature is not a single value but a carefully selected range, determined primarily by the material's melting point and the desired final properties of the part, such as density and strength. As a fundamental rule, the ideal temperature for solid-state sintering typically falls between 50% and 90% of the material's absolute melting temperature (measured in Kelvin).
The core challenge in determining sintering temperature is finding the "sweet spot" that provides enough thermal energy for atoms to bond particles together (densification), but not so much that it causes melting, distortion, or undesirable grain growth that weakens the final product.
The Core Principle: Activating Atomic Diffusion
Sintering works by heating a compacted powder to a point where atoms can move, or diffuse, across the boundaries of the particles. This atomic movement closes the pores between particles, fusing them into a solid, dense mass. Temperature is the primary catalyst for this process.
The Role of Melting Point (T_m)
The most critical factor governing sintering temperature is the material's melting point. The widely accepted range of 0.5 to 0.9 T_m (in Kelvin) defines the window where solid-state diffusion becomes significant enough to bond particles without causing them to melt.
Below this range, atomic movement is too slow for effective densification. Above it, you risk entering a liquid phase, which fundamentally changes the process and can ruin the part's shape.
Material Composition and Powder Characteristics
The specific "powder type" dictates its diffusion behavior. Metals, with their metallic bonds, generally allow for easier atom movement compared to ceramics, which have strong covalent or ionic bonds and often require higher relative temperatures.
Furthermore, smaller particle sizes possess higher surface energy. This acts as a driving force for sintering, often allowing for effective densification at lower temperatures or in shorter times compared to coarser powders.
The Sintering Atmosphere
The gaseous environment inside the furnace is also a critical parameter. A vacuum or an inert gas (like argon) is often used to prevent oxidation, which can inhibit proper bonding. In some cases, a reactive atmosphere is used to achieve specific chemical changes during sintering.
Aligning Temperature with Desired Outcomes
The choice of temperature within the sintering window is a strategic decision based on the final product's requirements.
Maximizing Density and Strength
To achieve the highest possible density and mechanical strength, engineers typically push the temperature toward the upper end of the sintering window. Higher heat accelerates diffusion, leading to more complete elimination of pores and stronger bonds between particles.
Maintaining Dimensional Accuracy
If precise final dimensions are the main goal, a lower temperature may be preferred. Sintering always involves shrinkage, and higher temperatures cause more rapid and sometimes less predictable shrinkage. Using a lower temperature slows this process, allowing for greater control over the final part's geometry.
Controlling Grain Size
Temperature has a direct impact on the final microstructure, specifically grain size. High temperatures and long hold times promote grain growth, where smaller grains merge into larger ones. While this aids densification, excessively large grains can make a material more brittle. For applications requiring toughness, a lower temperature is often used to maintain a fine-grained structure.
Understanding the Trade-offs
Selecting a sintering temperature is a balancing act with clear consequences for getting it wrong.
The Risk of Under-Sintering (Too Low)
If the temperature is too low, atomic diffusion will be insufficient. The resulting part will be highly porous, mechanically weak, and may not have fused into a coherent object. This is often called a "green" or "brown" part.
The Risk of Over-Sintering (Too High)
Exceeding the optimal temperature can be catastrophic. The material may begin to melt, causing the part to slump, distort, or lose its shape entirely. It also leads to rapid grain growth, which can severely degrade mechanical properties like toughness and fatigue resistance.
The Time-Temperature Relationship
Time and temperature are interdependent variables. You can often achieve a similar level of densification by using a lower temperature for a longer period. This relationship gives process engineers flexibility to optimize for specific outcomes, such as minimizing energy costs or controlling grain size.
Making the Right Choice for Your Goal
Ultimately, determining the precise temperature is a combination of theoretical knowledge and empirical testing.
- If your primary focus is maximum strength and density: Aim for the higher end of the material's sintering window (e.g., 0.8-0.9 T_m), but monitor the microstructure to prevent excessive grain growth.
- If your primary focus is high precision and dimensional control: Use the lower end of the sintering window (e.g., 0.5-0.7 T_m) and consider extending the hold time to achieve the necessary density.
- If you are working with a new material or alloy: Begin with theoretical calculations, consult phase diagrams to identify the solidus temperature, and use experimental methods like dilatometry to pinpoint the active densification range before performing iterative tests.
Mastering sintering temperature is about balancing atomic energy with structural control to achieve your specific engineering goal.

Summary Table:
| Factor | Impact on Sintering Temperature |
|---|---|
| Material Melting Point (T_m) | Primary factor; sets the range (0.5-0.9 T_m in Kelvin). |
| Desired Density/Strength | Higher temperatures (upper end of range) maximize density. |
| Dimensional Accuracy | Lower temperatures (lower end of range) improve control. |
| Powder Particle Size | Smaller particles can sinter effectively at lower temperatures. |
| Atmosphere (e.g., Vacuum) | Prevents oxidation, allowing for proper bonding at target temperature. |
Achieve precise sintering results for your lab. Selecting the correct temperature is critical for producing strong, durable, and dimensionally accurate sintered parts. KINTEK specializes in providing the high-quality lab furnaces and expert support you need to perfect your sintering process. Contact our experts today to discuss your specific materials and application goals.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the minimum temperature for a muffle furnace? Understanding Its High-Tech Design
- What is a muffle furnace test? Achieve Precise, Contamination-Free Heating for Your Lab
- What is the principle of muffle furnace? Achieve Pure, Precise High-Temperature Heating
- How do you maintain a muffle furnace? Ensure Safety and Maximize Equipment Lifespan
- What is the most common form of heat treatment? Mastering Annealing, Hardening, and Tempering



















