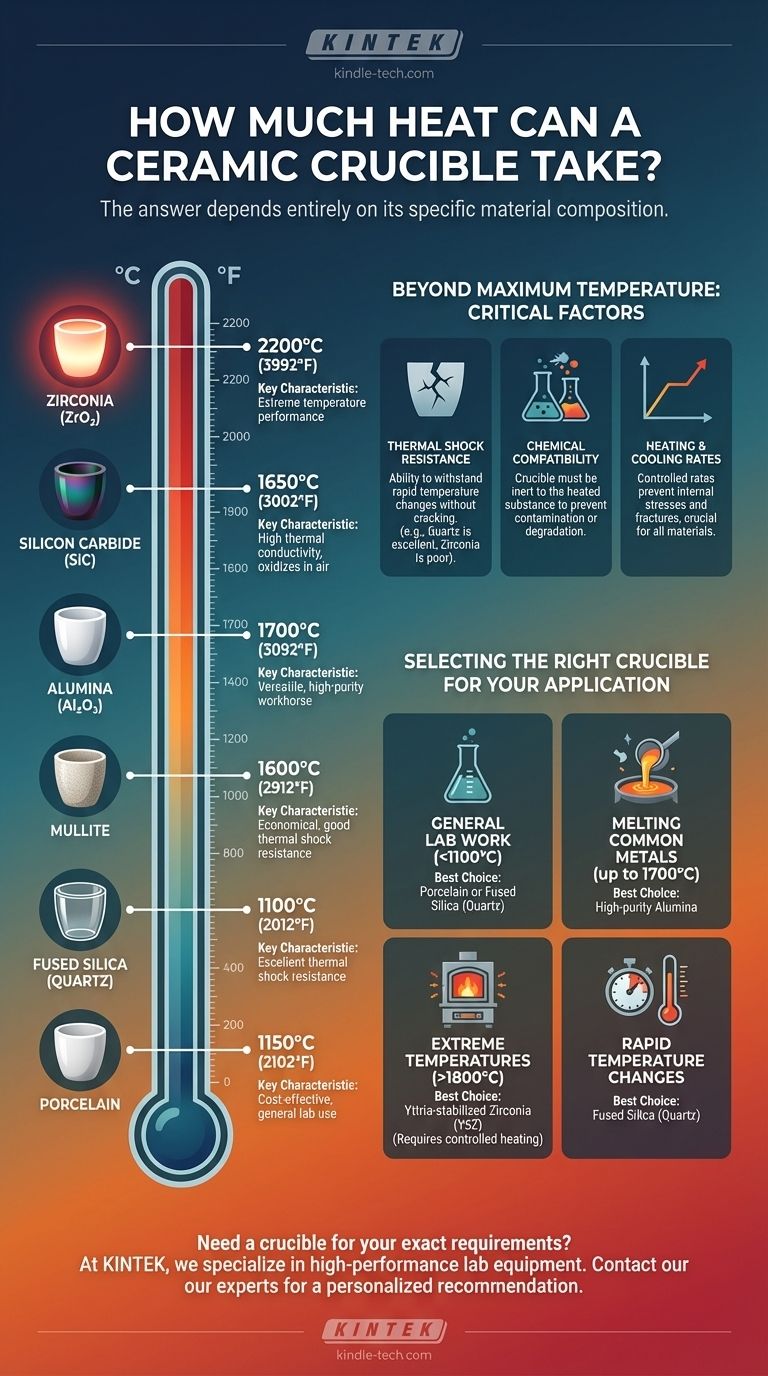
The maximum temperature a ceramic crucible can withstand depends entirely on its specific material composition. While a simple porcelain crucible may fail above 1200°C (2192°F), a high-purity zirconia crucible can operate effectively at temperatures well over 2000°C (3632°F). Therefore, identifying the exact type of ceramic is the critical first step.
The most important takeaway is that "ceramic" is a broad category, not a single material. The key to success is matching the specific ceramic—be it alumina, zirconia, or quartz—to your target temperature, heating rate, and the chemical nature of the material you are working with.

Why "Ceramic" Is Not a Single Answer
The term "ceramic" refers to a wide class of inorganic, non-metallic materials. Their properties, especially heat resistance, are determined by their unique chemical makeup and crystalline structure.
The Importance of Material Composition
Each ceramic compound has a different melting point and different characteristics under thermal stress. A crucible made of aluminum oxide will behave very differently from one made of zirconium dioxide at the same high temperature.
Common High-Temperature Ceramic Materials
Understanding the limits of the most common materials is the best way to determine which crucible you need.
- Porcelain: This is a cost-effective choice for general laboratory use. It has a typical maximum use temperature of around 1150°C (2102°F), though some variants can go slightly higher.
- Fused Silica (Quartz): Known for its exceptional resistance to thermal shock (cracking from rapid temperature changes). Its maximum continuous operating temperature is lower, around 1100°C (2012°F).
- Alumina (Al₂O₃): This is the most common and versatile high-temperature technical ceramic. High-purity alumina (99%+) crucibles can be used continuously at temperatures up to 1700°C (3092°F).
- Mullite (3Al₂O₃·2SiO₂): An excellent and economical alternative to alumina, mullite offers great strength at high temperatures and good thermal shock resistance. Its maximum use temperature is around 1600°C (2912°F).
- Zirconia (ZrO₂): For applications requiring even higher temperatures, yttria-stabilized zirconia (YSZ) is the standard. It has a very high melting point and can operate at temperatures up to 2200°C (3992°F).
- Silicon Carbide (SiC): This material offers excellent thermal shock resistance and high thermal conductivity. It is often used in applications where heat needs to be transferred quickly and can be used up to 1650°C (3002°F), though it can oxidize in air.
Beyond Maximum Temperature: Critical Factors for Success
Simply knowing the melting point is not enough. True crucible failure often happens for reasons other than just exceeding the maximum temperature.
Thermal Shock Resistance
This is a material's ability to withstand rapid changes in temperature without cracking. A crucible with poor thermal shock resistance can shatter if heated or cooled too quickly. Quartz is excellent in this regard, while zirconia is generally poor.
Chemical Compatibility
At high temperatures, chemical reactions accelerate. The material inside your crucible can react with the crucible itself, leading to contamination of your sample or degradation of the crucible. Always verify that your crucible material is inert to the substance you are heating.
Heating and Cooling Rates
Even for materials with good thermal shock resistance, a controlled heating and cooling rate is crucial. A slow, steady ramp-up and ramp-down in temperature prevents the buildup of internal stresses that can cause fractures, especially in larger or thicker-walled crucibles.
Understanding the Trade-offs
Choosing a crucible always involves balancing competing factors. There is no single "best" material for all situations.
Cost vs. Performance
There is a direct correlation between temperature performance and price. Porcelain is inexpensive, alumina represents a mid-range workhorse, and high-purity zirconia for extreme temperatures is a significant investment.
Thermal Shock vs. Max Temperature
Often, the materials with the highest temperature ratings (like zirconia) have the poorest thermal shock resistance. Conversely, fused silica (quartz) has phenomenal thermal shock resistance but a much lower maximum operating temperature.
Purity and Contamination
For high-purity applications, such as growing crystals or analyzing trace elements, the purity of the crucible itself is paramount. A lower-purity crucible can leach impurities into your melt, compromising the results.
Selecting the Right Crucible for Your Application
To make the correct choice, match the material to your specific process parameters.
- If your primary focus is general lab work or ashing below 1100°C: Porcelain or fused silica (quartz) are excellent, cost-effective choices.
- If your primary focus is melting common metals like gold, copper, or steel alloys (up to 1700°C): High-purity alumina is the industry standard for reliability and performance.
- If your primary focus is working with specialty alloys or materials above 1800°C: Yttria-stabilized zirconia (YSZ) is the necessary choice, but requires very careful and controlled heating protocols.
- If your primary focus is a process involving rapid temperature changes: Fused silica (quartz) is superior for its thermal shock resistance, provided you stay within its temperature limits.
Choosing the right crucible is about matching the material's specific properties to the precise thermal and chemical demands of your process.
Summary Table:
| Ceramic Material | Max Temperature (°C) | Max Temperature (°F) | Key Characteristics |
|---|---|---|---|
| Porcelain | 1150°C | 2102°F | Cost-effective, general lab use |
| Fused Silica (Quartz) | 1100°C | 2012°F | Excellent thermal shock resistance |
| Alumina (Al₂O₃) | 1700°C | 3092°F | Versatile, high-purity workhorse |
| Mullite | 1600°C | 2912°F | Economical, good thermal shock resistance |
| Zirconia (ZrO₂) | 2200°C | 3992°F | Extreme temperature performance |
| Silicon Carbide (SiC) | 1650°C | 3002°F | High thermal conductivity, oxidizes in air |
Need a crucible that matches your exact temperature and chemical requirements? At KINTEK, we specialize in high-performance lab equipment and consumables, including a full range of ceramic crucibles made from alumina, zirconia, quartz, and more. Our experts can help you select the perfect crucible for your specific application—ensuring safety, purity, and efficiency in your high-temperature processes. Contact our team today for a personalized recommendation and get the right solution for your laboratory needs!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
People Also Ask
- How does the use of corrosion-resistant ceramic crucibles ensure the chemical purity of materials? | KINTEK
- What are the functional advantages of using high-purity alumina crucibles? Achieve Precise Oxidation Data
- Why is a high-purity alumina crucible preferred for high-temperature oxidation? Ensure Unmatched Data Integrity
- What are the advantages of high-purity alumina crucibles for molten ZnNaK//Cl salts? Ensure Experimental Purity
- What is the function of alumina crucibles in Na3V2(PO4)2F3 synthesis? Ensure Purity in NVPF Production



















