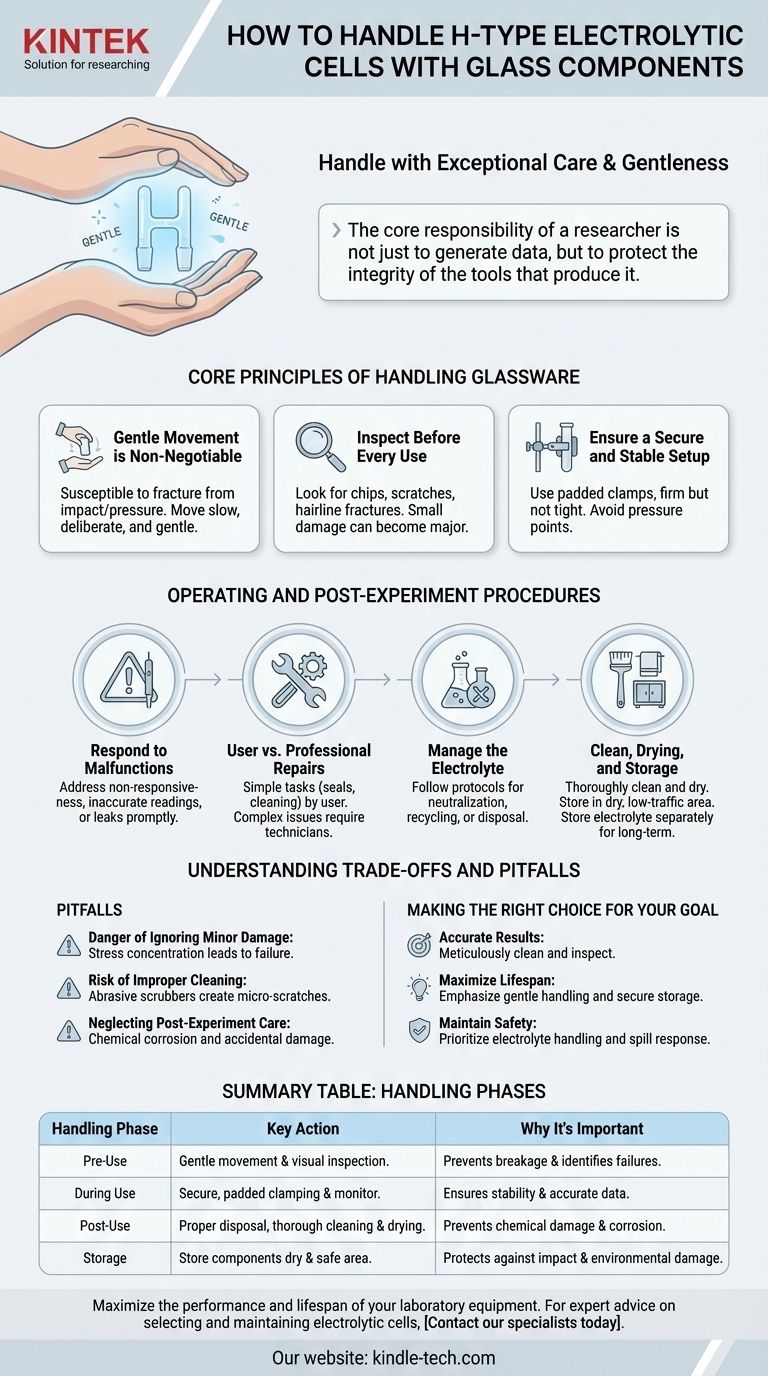
To properly handle an H-type electrolytic cell, you must treat its glass components with exceptional care and gentleness at all times. This fundamental principle of careful movement is the first step in preventing breakage and ensuring the equipment's longevity. Proper handling, however, extends beyond just physical movement to include post-experiment procedures and storage.
The core responsibility of a researcher is not just to generate data, but to protect the integrity of the tools that produce it. Proper handling of a glass electrolytic cell is a holistic process that encompasses pre-use inspection, operational awareness, and meticulous post-experiment care to ensure both safety and reliable results.

Core Principles of Handling Glassware
The inherent fragility of glass demands a disciplined and consistent approach. Overlooking these fundamental principles is the most common cause of equipment failure.
Gentle Movement is Non-Negotiable
Because the cell is made of glass, it is highly susceptible to fracture from impact or pressure. Always lift, move, and place the cell and its components with slow, deliberate, and gentle motions.
Inspect Before Every Use
Before assembling the cell, perform a quick visual inspection. Look for any new chips, scratches, or hairline fractures, especially around joints or ports. A small, seemingly insignificant point of damage can become a major failure point under thermal or mechanical stress.
Ensure a Secure and Stable Setup
When mounting the cell in your experimental apparatus, ensure that clamps are firm but not overly tight. Use clamps with soft, non-abrasive padding to avoid creating pressure points on the glass that could lead to fractures.
Operating and Post-Experiment Procedures
Safe and effective use of the cell requires attention to detail during and after the experiment. Your actions directly impact the equipment's lifespan and the quality of your future work.
Responding to Malfunctions
If you observe a malfunction, such as a non-responsive electrode, inaccurate temperature reading, or electrolyte leakage, the issue must be addressed promptly. Continuing an experiment with faulty equipment risks inaccurate data and potential damage.
Differentiating User vs. Professional Repairs
Simple faults, like replacing a worn sealing ring or cleaning a fouled electrode, can typically be performed by the user. For more complex problems, such as internal component damage or persistent leaks, you must contact a professional repair technician to avoid causing further damage.
Managing the Electrolyte
After the experiment concludes, the electrolyte must be handled according to its specific chemical properties. This involves following established protocols for neutralization, recycling, or certified disposal to prevent environmental harm.
Cleaning, Drying, and Storage
Thoroughly clean and dry the electrodes and the glass vessel after each use. Store all components in a dry, low-traffic environment to protect them from moisture and accidental impact.
For long-term storage, pour the electrolyte out of the cell and store it in a separate, properly sealed container. This prevents degradation of both the electrolyte and the cell components.
Understanding the Trade-offs and Pitfalls
While glass offers excellent chemical resistance and transparency, its primary trade-off is its fragility. Avoiding common mistakes is critical to mitigating this risk.
The Danger of Ignoring Minor Damage
A small chip might seem harmless, but it concentrates stress on the glass. Changes in temperature or even slight physical pressure can cause this weak point to propagate into a catastrophic crack, ruining an experiment and the equipment.
Risk of Improper Cleaning
Using abrasive scrubbers or harsh cleaning compounds can create micro-scratches on the glass surface. These scratches not only compromise the optical clarity but also act as stress concentrators, increasing the risk of future breakage.
Neglecting Post-Experiment Care
Failing to properly clean, dry, and store the cell is a common pitfall. Residual chemicals can etch the glass or corrode electrodes over time, while improper storage leaves the fragile components vulnerable to accidental damage.
Making the Right Choice for Your Goal
Your handling protocol should align with your primary objective. Different priorities require focusing on different aspects of the process.
- If your primary focus is ensuring accurate results: Meticulously clean all components after each use and inspect electrodes for fouling or damage before you begin.
- If your primary focus is maximizing equipment lifespan: Emphasize gentle physical handling, secure clamping, and proper long-term storage in a dry, safe location.
- If your primary focus is maintaining a safe lab environment: Prioritize the correct handling and disposal of electrolytes and have a clear plan for addressing any leaks or spills immediately.
Disciplined handling of your equipment is not a chore; it is a fundamental aspect of precise and reproducible scientific work.
Summary Table:
| Handling Phase | Key Action | Why It's Important |
|---|---|---|
| Pre-Use | Gentle movement; visual inspection for damage | Prevents breakage and identifies potential failure points |
| During Use | Secure, padded clamping; monitor for malfunctions | Ensures stable operation and accurate data collection |
| Post-Use | Proper electrolyte disposal; thorough cleaning and drying | Prevents chemical damage and corrosion |
| Storage | Store components dry and in a safe, low-traffic area | Protects against accidental impact and environmental damage |
Maximize the performance and lifespan of your laboratory equipment. Proper handling is crucial, but so is having reliable, high-quality apparatus. KINTEK specializes in precision lab equipment and consumables, serving all your laboratory needs.
For expert advice on selecting and maintaining electrolytic cells and other essential lab tools, contact our specialists today to ensure your research is built on a foundation of quality and reliability.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide