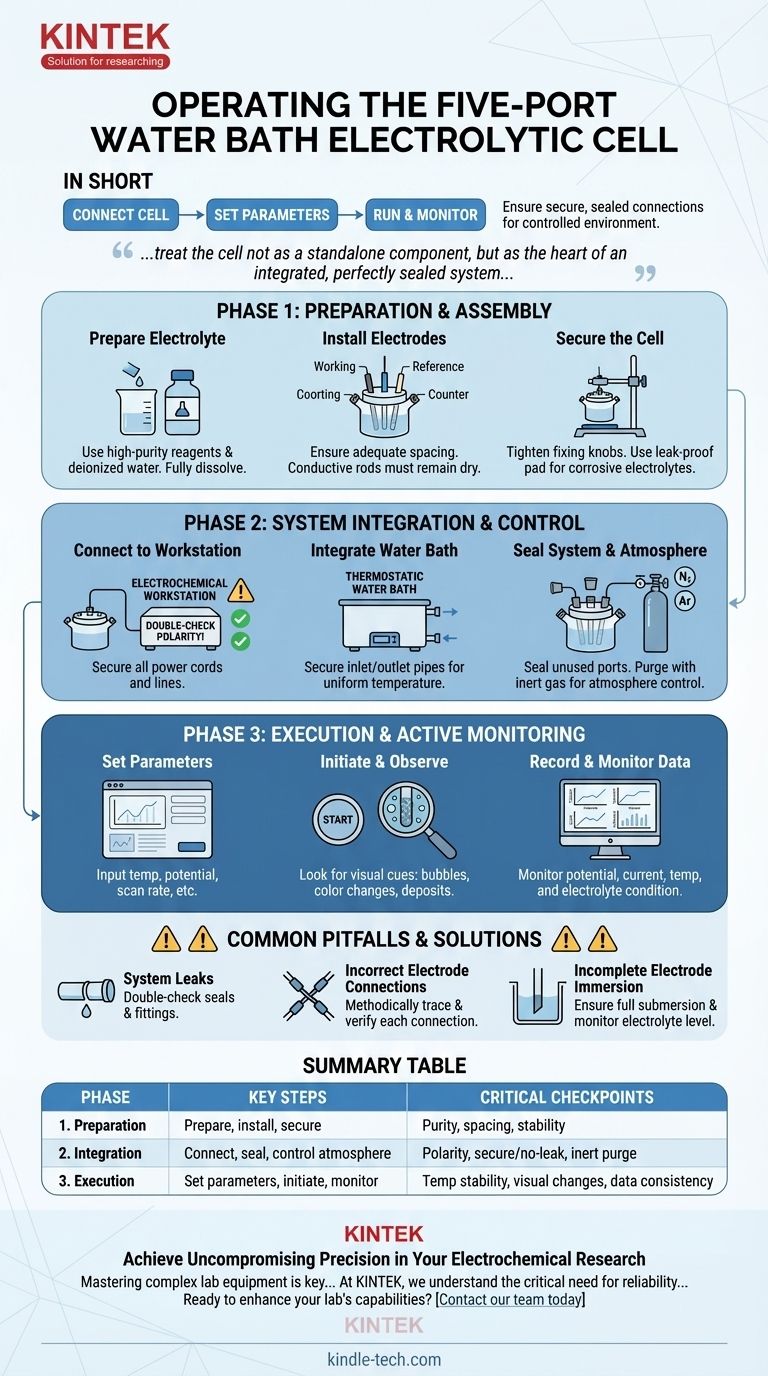
In short, operating a five-port water bath electrolytic cell involves a sequence of connecting the cell to your workstation and water bath, setting the required temperature and electrochemical parameters, running the experiment, and actively monitoring all reactions and data. The success of your experiment hinges on ensuring every connection is secure and perfectly sealed to maintain the integrity of your controlled environment.
The five-port electrolytic cell is designed for precision, giving you control over temperature and atmosphere. However, this control is only as good as your setup. The core principle is to treat the cell not as a standalone component, but as the heart of an integrated, perfectly sealed system where every connection and parameter is meticulously managed.

Phase 1: Preparation and Assembly
Before connecting any electronics, the physical and chemical foundation of your experiment must be perfect. This phase is about eliminating variables that could compromise your results.
Preparing the Electrolyte
The purity of your electrolyte directly impacts the accuracy of your experiment. Use high-purity chemical reagents and deionized or distilled water to prevent unwanted side reactions or contamination.
Prepare the solution according to your experimental protocol, ensuring all components are fully dissolved.
Installing the Electrodes
Carefully install your working, reference, and counter electrodes into the appropriate ports. Ensure there is adequate spacing between them to prevent short-circuiting and to allow for uniform current distribution.
Add the prepared electrolyte to the cell. The electrodes must be sufficiently immersed to cover the active surface area, but the conductive connecting rods at the top must remain dry and above the liquid level.
Securing the Cell
Place the electrolytic cell onto a stable laboratory stand and tighten any fixing knobs to ensure it is held vertically and cannot wobble. For corrosive electrolytes, placing a leak-proof pad underneath the cell is a critical safety precaution.
Phase 2: System Integration and Control
This phase involves connecting the cell to your external equipment. The integrity of these connections is paramount for controlling the experimental environment.
Connecting to the Electrochemical Workstation
Connect the electrode leads to the corresponding terminals on your electrochemical workstation. Double-check the polarity: connecting the positive and negative terminals incorrectly can damage your cell or invalidate your results.
Ensure all power cords and connection lines are intact and securely fastened to prevent data signal loss or electrical hazards.
Integrating with the Thermostatic Water Bath
The water bath is your key to precise temperature control. Connect the inlet and outlet pipes from the water bath to the designated ports on the cell's outer jacket.
Confirm that the connections are secure to prevent water leaks and to ensure proper circulation, which is essential for maintaining a uniform temperature throughout the cell.
Sealing the System and Controlling Atmosphere
All unused ports must be sealed with stoppers to create a closed system. This is non-negotiable if you need to control the gaseous atmosphere.
If your experiment requires an inert atmosphere (e.g., nitrogen or argon), purge the sealed cell with the gas before and sometimes during the experiment to remove oxygen and other contaminants. The fifth port is often used for this gas inlet/outlet.
Phase 3: Execution and Active Monitoring
With the system assembled and sealed, you are ready to begin the experiment. This is an active process, not a "set it and forget it" procedure.
Setting Experimental Parameters
Set the desired temperature on the thermostatic water bath and allow the system to equilibrate.
On the electrochemical workstation, input the necessary parameters for your experiment, such as the potential range, scan rate, or constant current/voltage.
Initiating the Experiment and Observing
Begin the experiment via the workstation software. Immediately begin observing the electrode surfaces for key phenomena.
Look for visual cues like gas bubble evolution, color changes in the electrolyte, or the formation of deposits on the working electrode. These qualitative observations are valuable data points.
Recording and Monitoring Data
Your workstation will record quantitative data like potential, current, and time. Simultaneously, you should monitor the temperature to ensure it remains stable.
Keep a close eye on the overall condition of the electrolyte and the system. Promptly address any abnormalities, such as unexpected leaks, temperature fluctuations, or erratic electrochemical signals.
Understanding the Common Pitfalls
Even with a perfect procedure, issues can arise. Being aware of common failure points is key to troubleshooting and ensuring data integrity.
Pitfall: System Leaks (Liquid or Gas)
A liquid leak from the water bath or electrolyte is a safety hazard and can damage equipment. A gas leak compromises your atmospheric control, introducing contaminants like oxygen that can ruin sensitive experiments. Solution: Double-check all seals and tube fittings before every experiment.
Pitfall: Incorrect Electrode Connections
Reversing the polarity of your electrode leads is a common mistake that can lead to completely incorrect data, irreversible damage to your reference electrode, or failure of the experiment. Solution: Methodically trace and verify each connection from the electrode to the workstation port before starting.
Pitfall: Incomplete Electrode Immersion
If the electrolyte level is too low or evaporates during a long experiment, the active surface area of your electrode changes. This invalidates current density calculations and makes your results non-repeatable. Solution: Ensure electrodes are fully submerged at the start and monitor the electrolyte level during long-duration runs.
Making the Right Choice for Your Goal
The way you operate the cell should be tailored to your specific research objective.
- If your primary focus is precise kinetic analysis: Your highest priority is maintaining a rock-solid temperature and a perfectly sealed, inert atmosphere.
- If your primary focus is electrodeposition or synthesis: Pay close attention to visual changes on the electrode surface and ensure consistent electrolyte concentration.
- If your primary focus is gas evolution reactions (e.g., HER/OER): The integrity of your gas-tight seal is paramount for accurate product collection and analysis.
Ultimately, mastering the five-port electrolytic cell is about exercising complete and repeatable control over your electrochemical environment.
Summary Table:
| Phase | Key Steps | Critical Checkpoints |
|---|---|---|
| 1. Preparation | Prepare electrolyte, install electrodes, secure cell. | Electrolyte purity, electrode spacing, cell stability. |
| 2. Integration | Connect to workstation & water bath, seal system, control atmosphere. | Correct polarity, secure/no-leak connections, inert gas purge. |
| 3. Execution | Set parameters, initiate experiment, actively monitor. | Temperature stability, visual electrode changes, data consistency. |
Achieve Uncompromising Precision in Your Electrochemical Research
Mastering complex lab equipment is key to breakthrough discoveries. At KINTEK, we understand the critical need for reliability and control in every experiment.
We specialize in providing high-quality laboratory equipment and consumables, including electrochemical cells and workstations, designed for accuracy and durability. Let our experts help you equip your lab for success.
Ready to enhance your lab's capabilities? Contact our team today to find the perfect solutions for your research needs!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis



















