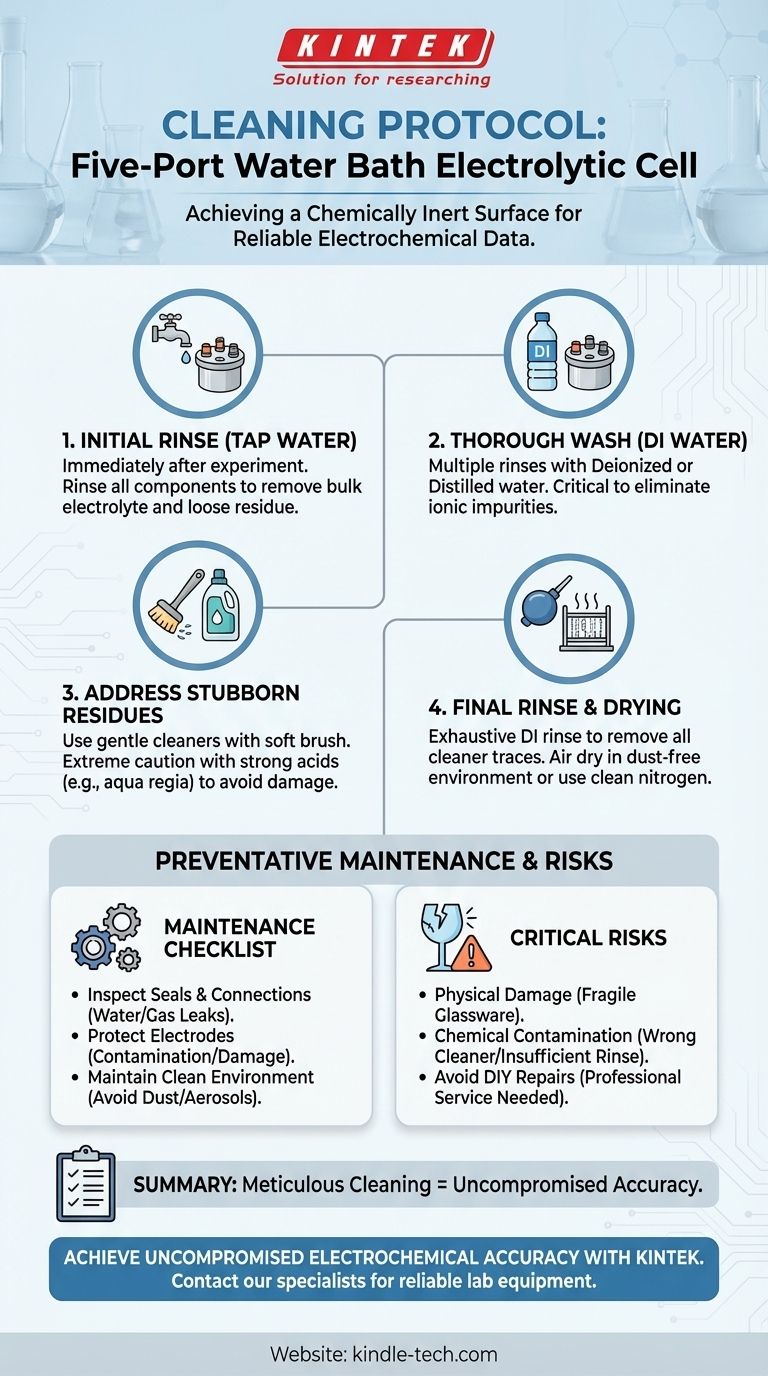
To properly clean a five-port water bath electrolytic cell, you must follow a systematic procedure of rinsing, washing, and final rinsing to eliminate contaminants without damaging the fragile glassware. Begin by rinsing all components with tap water to remove loose electrolyte, followed by multiple rinses with deionized or distilled water. For stubborn residues, a carefully selected cleaning agent may be used, but this requires extreme caution to avoid scratching the glass or introducing chemical impurities.
The core principle of cleaning an electrolytic cell is not just about visible cleanliness, but about achieving a chemically inert surface. The ultimate goal is to eliminate any source of contamination that could compromise the accuracy and reproducibility of your electrochemical measurements.

The Standard Cleaning Protocol: A Step-by-Step Guide
Proper cleaning is foundational to reliable electrochemical data. Each step is designed to remove specific types of residue while protecting the cell's integrity.
Step 1: Initial Rinse with Tap Water
Immediately after your experiment, disassemble the cell. Rinse the cell body, stoppers, Luggin capillary, and any other components with tap water. This initial step effectively removes the bulk of the residual electrolyte and any loosely adhered products.
Step 2: Thorough Wash with Deionized Water
After the tap water rinse, wash all parts multiple times with deionized (DI) or distilled water. This is a critical step, as it removes the ionic impurities present in tap water. The goal is to leave the surface free of any ions that could interfere with subsequent experiments.
Step 3: Addressing Stubborn Residues
For persistent stains or adsorbed chemical species, a more aggressive cleaning agent may be necessary. Use a soft brush or cloth to apply the cleaner, avoiding any abrasive action that could scratch the glass.
The choice of cleaner is critical. For general organic residues, a solution like Alconox may be sufficient. For stubborn metallic or organic deposits, chemists may resort to powerful solutions like aqua regia or piranha solution, but these are extremely hazardous and should only be used with proper safety protocols and a deep understanding of their chemical reactivity.
Step 4: Final Rinse and Drying
After using any cleaning agent, you must perform an exhaustive final rinse with DI water to remove every trace of the cleaner. Any remaining cleaning agent will act as a contaminant in your next experiment.
Allow the components to air dry completely in a dust-free environment or use a stream of clean, dry nitrogen.
Beyond Cleaning: Essential Preventative Maintenance
Consistent results depend on more than just post-experiment cleaning. Proactive maintenance is key to the cell's long-term performance and reliability.
Inspecting Seals and Connections
Regularly check the seals on both the water bath circulation system and the cell's ports. Degraded or loose seals can lead to water or gas leaks, which can ruin an experiment and potentially damage your equipment. Ensure all PTFE stoppers form a tight seal.
Protecting the Electrodes
Your electrodes are the most sensitive part of the setup. Always inspect their surfaces for contamination, pitting, or physical damage before each use. Avoid leaving electrodes exposed to air or soaking in solution for prolonged periods when not in use, as this can lead to oxidation or deterioration.
Maintaining a Clean Experimental Environment
An electrolytic cell is highly susceptible to environmental contamination. Avoid performing any operations that generate dust, aerosols, or other pollutants near your experimental setup. Keeping the surrounding area clean is a simple but effective way to protect the purity of your electrolyte.
Understanding the Risks and Limitations
Improper maintenance introduces significant risks that can invalidate your work. Understanding these pitfalls is as important as knowing the cleaning procedure itself.
The Risk of Physical Damage
The cell body is made of glass and is inherently fragile. Always handle it gently. Applying excessive force, using abrasive cleaning tools, or causing thermal shock can lead to scratches or fractures, rendering the cell useless.
The Danger of Chemical Contamination
The most insidious risk is chemical contamination. Using the wrong cleaning agent can etch the glass or introduce impurities. More commonly, insufficient rinsing after cleaning leaves a film of residue that will interfere with your electrochemical measurements, leading to skewed data and non-reproducible results.
When to Avoid DIY Repairs
While routine cleaning is your responsibility, certain problems require professional service. Do not attempt to repair a malfunctioning water bath circulator, damaged electrical connections, or severely compromised seals yourself. This can lead to further damage and safety hazards.
Making the Right Choice for Your Experiment
Your cleaning and maintenance strategy should be adapted to the sensitivity of your work.
- If your primary focus is trace analysis or high-sensitivity measurements: Meticulous cleaning with ultra-pure water is non-negotiable, and you may need to dedicate specific glassware to avoid cross-contamination.
- If your primary focus is routine voltammetry or general synthesis: The standard cleaning protocol is sufficient, but consistency is crucial for reproducibility between experiments.
- If you are working with persistent organic or metallic films: You may require aggressive cleaning agents, but you must understand the chemistry and accept the need for an exhaustive rinsing protocol.
Ultimately, diligent and thoughtful maintenance is the bedrock upon which reliable electrochemical data is built.
Summary Table:
| Step | Action | Purpose | Key Consideration |
|---|---|---|---|
| 1 | Initial Rinse with Tap Water | Remove bulk electrolyte and loose residue | Perform immediately after experiment |
| 2 | Wash with Deionized Water | Eliminate ionic impurities from tap water | Multiple rinses are critical |
| 3 | Address Stubborn Residues | Remove persistent stains or films | Use gentle cleaners; avoid abrasives |
| 4 | Final Rinse & Drying | Ensure no cleaner residue remains | Air dry or use dry nitrogen stream |
Achieve Uncompromised Electrochemical Accuracy with KINTEK
Your research depends on the integrity of your lab equipment. KINTEK specializes in high-precision laboratory equipment and consumables, ensuring your electrolytic cells and other critical apparatus deliver reliable, contamination-free performance.
We understand that consistent, reproducible results are paramount. Whether you are engaged in trace analysis, routine voltammetry, or complex synthesis, the right equipment and maintenance practices are foundational.
Let us help you safeguard your experiments:
- Source reliable, chemically inert lab glassware designed to withstand rigorous cleaning protocols.
- Access high-purity consumables like deionized water and specialized cleaning agents.
- Get expert support on maintenance best practices to extend equipment life and data quality.
Don't let maintenance uncertainties compromise your data. Contact our lab equipment specialists today to discuss how KINTEK can support your laboratory's specific needs for precision and reliability.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results



















