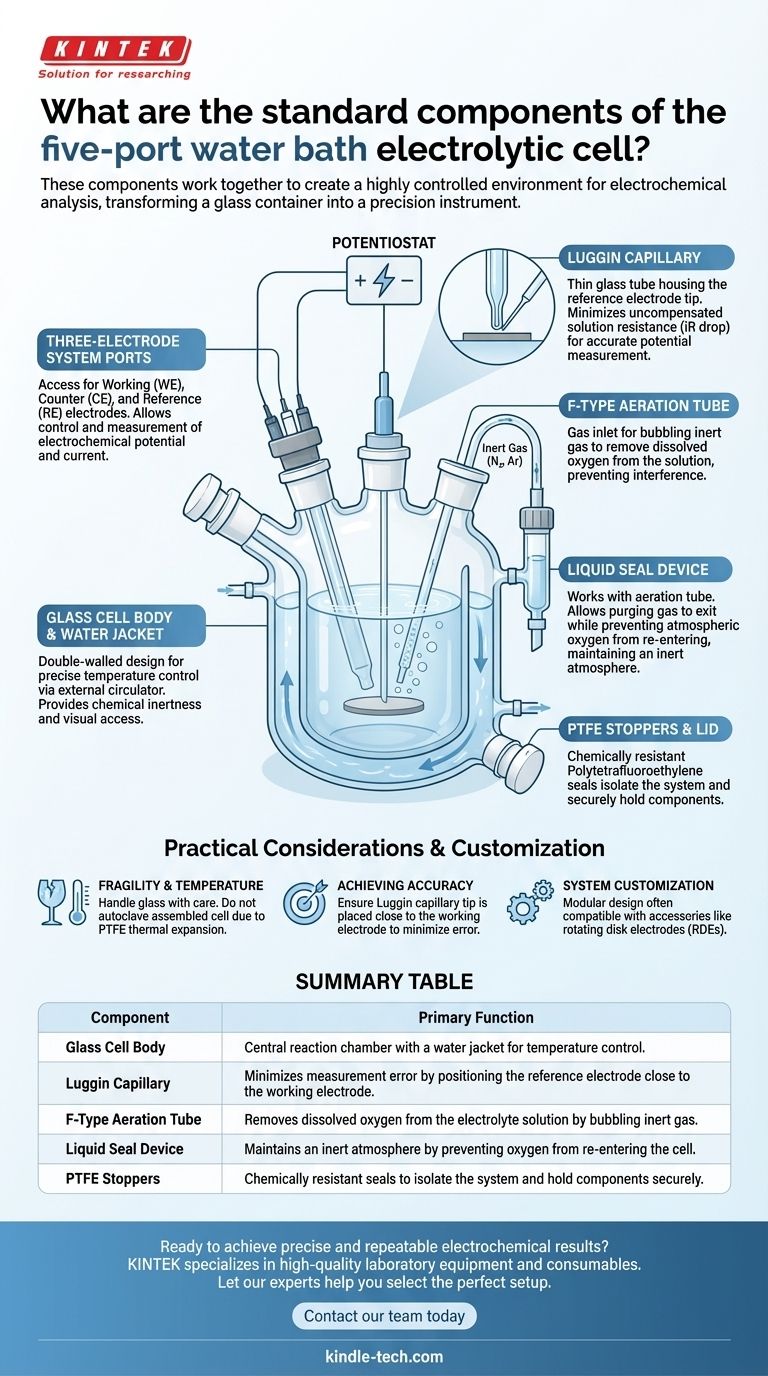
The standard components of a five-port water bath electrolytic cell are the glass cell body with its water jacket, an F-type aeration tube, a liquid seal device, a Luggin capillary, and Polytetrafluoroethylene (PTFE) stoppers to seal the ports. These components work together to create a highly controlled environment for electrochemical analysis.
This specialized cell is not merely a container, but a precision instrument. Understanding the distinct function of each part is essential for isolating variables and achieving accurate, repeatable experimental results.

The Core Components and Their Functions
A five-port electrolytic cell provides the necessary inputs and outputs to control the chemical environment while performing a three-electrode electrochemical experiment.
The Glass Cell Body
The cell body is the central reaction chamber that holds the electrolyte solution. It is typically made of glass to provide chemical inertness and visual access to the experiment.
These cells feature a double-walled "water jacket" design. This allows a fluid from a temperature-controlled circulator to flow around the main chamber, maintaining a precise and stable experimental temperature.
The Three-Electrode System
While the electrodes themselves are chosen based on the experiment, the cell is designed to hold them. The five ports provide access for a working electrode, a counter electrode (or auxiliary electrode), and a reference electrode.
The electrodes are connected to an external power source, called a potentiostat, which controls the potential and measures the resulting current.
The Luggin Capillary
The Luggin capillary is a crucial component for accuracy. It is a thin glass tube that houses the tip of the reference electrode, allowing it to be positioned extremely close to the surface of theworking electrode.
Its purpose is to minimize the uncompensated solution resistance (iR drop) between the working and reference electrodes. This ensures that the potential being measured is as accurate as possible, which is critical for many electrochemical studies.
The F-Type Aeration Tube
This is a gas inlet tube used to bubble an inert gas, such as nitrogen or argon, through the electrolyte solution before an experiment.
Its function is to remove dissolved oxygen from the solution. Oxygen is electrochemically active and can interfere with a wide range of experiments, so removing it is a standard and necessary step.
The Liquid Seal Device
The liquid seal works in tandem with the aeration tube. After purging the solution with inert gas, the gas flow is redirected to flow over the top of the solution and out through the seal.
This device allows the purging gas to exit while preventing atmospheric oxygen from re-entering the cell. This maintains a stable, inert atmosphere over the electrolyte for the duration of the experiment.
PTFE Stoppers and Lid
The remaining ports are sealed with chemically resistant Polytetrafluoroethylene (PTFE) stoppers or a larger PTFE lid. This isolates the system from the outside environment and holds the various components securely in place.
Practical Considerations and Common Pitfalls
Proper handling and awareness of material limitations are as important as understanding the function of each component.
Material Fragility and Temperature Limits
The glass cell body is fragile and must be handled with care to prevent breakage.
More importantly, the PTFE components have a different thermal expansion coefficient than glass. Do not heat or autoclave the entire cell assembly. The PTFE lid or stoppers can expand and deform permanently, ruining the seal. The glass body can be autoclaved separately if sterilization is required.
Achieving Accurate Measurements
The primary source of error in potential measurement is often a poorly placed reference electrode. Ensure the tip of the Luggin capillary is positioned as close as possible to the working electrode without touching it.
System Customization
These cells are often modular. The exact port configuration can sometimes be customized, and they are typically compatible with specialized accessories like rotating disk electrodes (RDEs) for hydrodynamic studies.
Assembling Your Cell for a Successful Experiment
Your experimental goal dictates which components require the most attention during setup.
- If your primary focus is high-precision potential measurement: Ensure the Luggin capillary tip is placed correctly to minimize iR drop.
- If your primary focus is studying oxygen-sensitive reactions: Use the aeration tube and liquid seal properly to thoroughly de-gas the electrolyte and maintain an inert atmosphere.
- If your primary focus is temperature-dependent studies: Connect the cell's water jacket to a stable, external water bath circulator to ensure precise temperature control.
By mastering the function of each part, you transform a piece of glassware into a reliable tool for discovery.
Summary Table:
| Component | Primary Function |
|---|---|
| Glass Cell Body | Central reaction chamber with a water jacket for temperature control. |
| Luggin Capillary | Minimizes measurement error by positioning the reference electrode close to the working electrode. |
| F-Type Aeration Tube | Removes dissolved oxygen from the electrolyte solution by bubbling inert gas. |
| Liquid Seal Device | Maintains an inert atmosphere by preventing oxygen from re-entering the cell. |
| PTFE Stoppers | Chemically resistant seals to isolate the system and hold components securely. |
Ready to achieve precise and repeatable electrochemical results? The right equipment is fundamental to your success. KINTEK specializes in high-quality laboratory equipment and consumables, including precision electrolytic cells and accessories designed for accuracy and durability. Let our experts help you select the perfect setup for your research needs.
Contact our team today to discuss your specific application and discover how KINTEK can support your laboratory's goals.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results



















