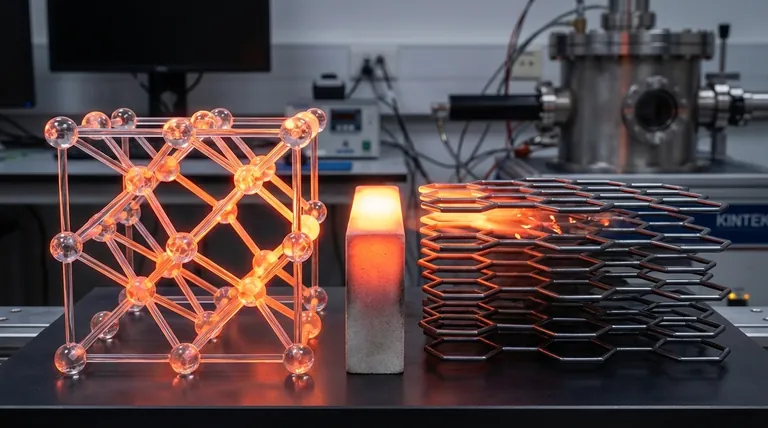
Yes, diamond is a vastly superior thermal conductor compared to graphite. While both are pure forms of carbon, diamond's unique atomic structure allows it to transfer heat with extreme efficiency, making it one of the best thermal conductors of any known material at room temperature. Its thermal conductivity can be over five times higher than that of copper and many times higher than graphite's.
The core reason for this difference lies not in their chemical makeup, but in their atomic architecture. Diamond's rigid, three-dimensional lattice is a highly efficient "superhighway" for heat energy, whereas graphite's layered structure creates significant roadblocks that impede thermal flow.
How Heat Travels in Solids: The Role of Phonons
What is a Phonon?
In an electrically insulating solid, heat is not carried by electrons but by lattice vibrations. Think of the atoms in the crystal as being connected by springs. A vibration at one end creates a wave that travels through the entire structure.
These quantized waves of atomic vibration are called phonons. The efficiency of heat conduction depends on how easily these phonons can travel through the material without being scattered or disrupted.
The Importance of a Stiff, Uniform Lattice
A perfect material for heat conduction has strong, stiff atomic bonds and a highly ordered, uniform structure. This allows vibrational energy (phonons) to propagate cleanly with minimal resistance.
Any irregularity, impurity, or weakness in the lattice acts as a scattering point, disrupting the flow of phonons and reducing thermal conductivity.
The Diamond Advantage: A Perfect Lattice for Heat Transfer
The sp³ Bonded Tetrahedral Structure
Each carbon atom in a diamond is bonded to four other carbon atoms in a tetrahedral arrangement. This sp³ bonding repeats in all three dimensions, creating an incredibly strong, rigid, and continuous cubic lattice.
This structure is what makes diamond the hardest known natural material. There are no weak points or planes within the crystal.
Why This Structure Excels at Phonon Transport
Diamond's rigid and perfectly uniform lattice is an ideal medium for phonon transport. The strong covalent bonds allow vibrational energy to travel at very high speeds with very little scattering.
This makes diamond an exceptional thermal conductor, with a conductivity around 2000 W/m·K. This is why diamond is used as a heat sink for high-power electronics where dissipating heat is critical.
The Graphite Limitation: A Story of Two Directions
The sp² Bonded Layered Structure
In graphite, each carbon atom is bonded to only three others in a flat, hexagonal sheet. This sp² bonding is very strong, but only within the two-dimensional plane of the sheet.
These sheets are stacked on top of each other and are held together by much weaker forces (van der Waals forces). This layered structure is what allows graphite to be brittle and act as a good lubricant, as the layers can easily slide past one another.
Anisotropic Conductivity: Fast Along the Sheets, Slow Between Them
This layered structure makes graphite's thermal conductivity anisotropic, meaning it is different in different directions.
Heat travels very efficiently along the hexagonal sheets, but it struggles to jump from one sheet to the next across the weak bonds. The weak interlayer connection acts as a major bottleneck for phonon transport.
As a result, graphite's overall thermal conductivity is significantly lower than diamond's, typically ranging from 200-500 W/m·K within the planes and far less between them.
Understanding the Trade-offs: Stability vs. Performance
The Paradox of Thermodynamic Stability
The references correctly note that at standard temperature and pressure, graphite is the more thermodynamically stable form of carbon. Diamond is technically metastable.
However, this thermodynamic stability has no bearing on its thermal performance. A material's properties are dictated by its structure, not its relative stability.
The Activation Energy Barrier
Diamond does not spontaneously turn into the more stable graphite because a very large activation energy barrier separates the two forms.
An immense amount of energy is required to break diamond's rigid sp³ bonds to allow them to re-form into graphite's sp² structure. This high barrier is what makes diamonds effectively permanent under normal conditions.
Making the Right Choice for Your Goal
When selecting a carbon allotrope, the application dictates the choice.
- If your primary focus is maximum thermal dissipation: Diamond is the unparalleled choice, used for high-performance heat sinks, cutting tools, and specialized electronic substrates.
- If your primary focus is cost-effective, directional heat spreading: Graphite sheets are excellent for moving heat laterally away from a source, a common strategy in consumer electronics like phones and laptops.
- If your primary focus is electrical conductivity or lubrication: Graphite is the superior option, as its delocalized electrons allow it to conduct electricity and its weak interlayer bonds allow it to act as a dry lubricant.
Ultimately, understanding the direct link between a material's atomic structure and its physical properties is the key to solving any engineering challenge.
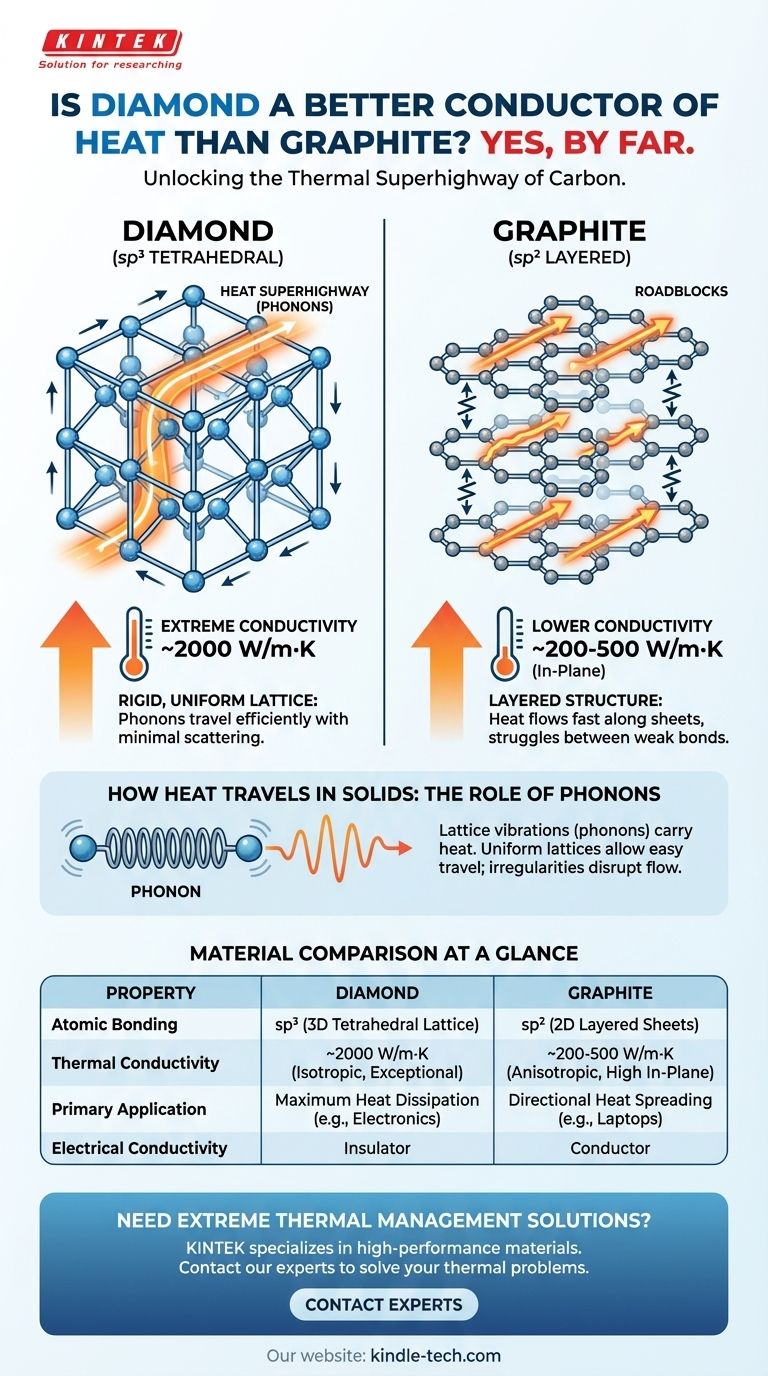
Summary Table:
| Property | Diamond | Graphite |
|---|---|---|
| Atomic Bonding | sp³ (3D tetrahedral lattice) | sp² (2D layered sheets) |
| Thermal Conductivity | ~2000 W/m·K (exceptional, isotropic) | 200-500 W/m·K (anisotropic, high in-plane) |
| Primary Application | Maximum heat dissipation (e.g., electronics) | Directional heat spreading (e.g., laptops) |
| Electrical Conductivity | Insulator | Conductor |
Need a material solution for extreme thermal management?
KINTEK specializes in high-performance lab equipment and materials, including advanced thermal solutions. Whether your project requires the unparalleled heat dissipation of diamond or the cost-effective, directional properties of graphite, our expertise can help you select the perfect material for your specific application.
Contact our experts today to discuss how we can enhance your laboratory's capabilities and solve your most challenging thermal problems.
Visual Guide
Related Products
- CVD Diamond for Thermal Management Applications
- CVD Diamond Domes for Industrial and Scientific Applications
- Silicon Carbide (SIC) Ceramic Sheet Flat Corrugated Heat Sink for Engineering Advanced Fine Ceramics
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- What is the fluorescence of a CVD diamond? A Guide to Its Unique Glow and Purpose
- What is the difference between CVD and original diamond? Choose the Right Diamond for Your Needs
- What is CVD diamond? The Ultimate Guide to Lab-Grown Diamonds and Their Uses
- What is the process of lab created diamonds? A Clear Guide to HPHT & CVD Methods
- Are CVD diamonds fake? Discover the Truth About Lab-Grown Diamonds