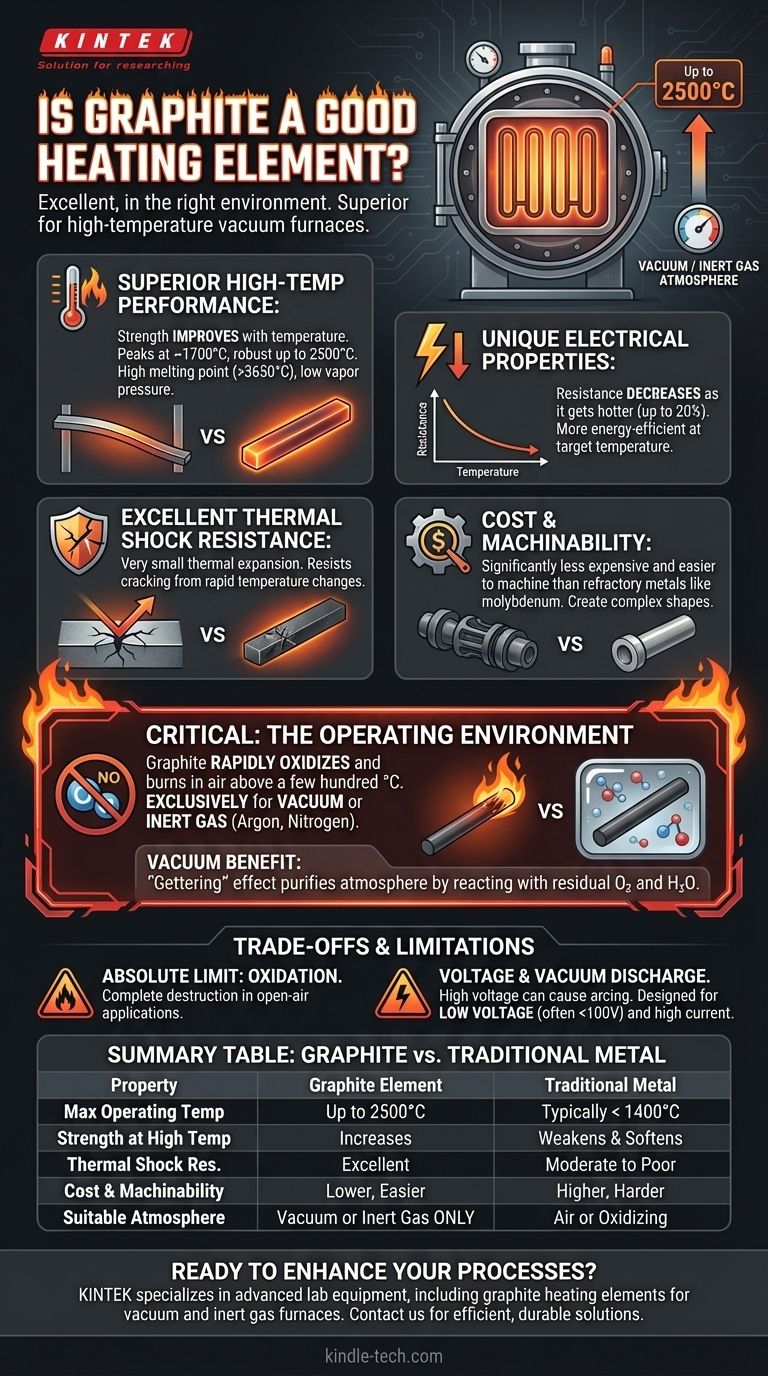
In the right environment, graphite is not just a good heating element; it is an excellent one. Its superiority comes from a unique combination of high-temperature strength, cost-effectiveness, and electrical properties that are fundamentally different from traditional metallic elements, making it an ideal choice for demanding applications like vacuum furnaces.
While many applications rely on metallic heaters, graphite excels in high-temperature, non-oxidizing environments. Its unique ability to grow stronger and more electrically conductive as it heats up makes it a more efficient and durable choice for specialized industrial processes, provided its operational limits are respected.

Why Graphite Excels as a Heating Element
Graphite's value is not universal; it is context-dependent. Its properties make it a dominant material in specific, high-performance applications where conventional metals would fail.
Superior High-Temperature Performance
Unlike metals, which weaken and soften as they approach their melting point, graphite’s mechanical strength improves with temperature, peaking around 1700°C and remaining robust up to 2500°C.
It possesses an extremely high melting point (sublimating around 3650°C) and low vapor pressure, ensuring stability during intense heating cycles.
Unique Electrical Properties
Graphite exhibits a negative temperature coefficient of resistance. This means its electrical resistance decreases as it gets hotter (by up to 20%).
This characteristic makes it more energy-efficient, as it draws more power and generates heat more effectively at its target operating temperature.
Excellent Thermal Shock Resistance
Graphite has a very small coefficient of thermal expansion. It does not expand or contract significantly when heated or cooled.
This property gives it outstanding resistance to thermal shock, meaning it is far less likely to crack or fail during rapid temperature changes.
Cost and Machinability
Compared to refractory metals like molybdenum or tungsten, graphite is significantly less expensive and easier to machine. This allows for the creation of large or complex heating element shapes, reducing both initial and replacement costs.
The Critical Role of the Operating Environment
The decision to use graphite is defined almost entirely by its intended atmosphere. It is a specialized tool, not a universal solution.
The Necessity of a Non-Oxidizing Atmosphere
Graphite’s primary weakness is its reaction with oxygen. At high temperatures in the presence of air, it will rapidly oxidize and burn away.
For this reason, graphite heating elements are used exclusively in vacuum furnaces or environments flooded with an inert gas like argon or nitrogen.
A Self-Purifying Effect in Vacuums
In a vacuum furnace, graphite provides an added benefit. It reacts with residual oxygen and water vapor—impurities in the vacuum—to form carbon monoxide (CO) and hydrogen (H₂), which are then pumped out.
This "gettering" effect actively purifies the furnace atmosphere, simplifying the vacuum system design and improving the quality of the process.
Heat Transfer via Radiation
Graphite elements are excellent radiators of thermal energy. Furnaces using them are designed to leverage this radiation-dominant heat transfer, which is highly effective and uniform in a vacuum.
Understanding the Trade-offs and Limitations
To use graphite effectively is to understand its boundaries. Ignoring them leads to rapid failure.
Oxidation Is Its Absolute Limit
This cannot be overstated. Using a graphite heating element in an open-air application above a few hundred degrees Celsius will result in its complete and rapid destruction.
Voltage and Vacuum Discharge
In vacuum environments, a high voltage potential across the element can cause an electrical arc, or "vacuum discharge."
To prevent this, systems using graphite heaters are typically designed to operate at a low voltage (often under 100V) and high current.
Conductor vs. Insulator: The Importance of Form
A common point of confusion is graphite's dual role. A solid graphite rod is an excellent electrical and thermal conductor, which is why it works as a heating element.
However, graphite felt or rigid fiberboard is an excellent thermal insulator. This is because its fibrous form consists mostly of empty space, which traps heat effectively. It is critical to distinguish between graphite elements (conductors) and graphite insulation.
Making the Right Choice for Your Application
Your choice of heating element must be dictated by your operating conditions and performance goals.
- If your primary focus is high-temperature vacuum or inert gas furnaces: Graphite is very likely your best choice due to its superior strength, thermal stability, and cost-effectiveness.
- If your primary focus is heating in an open-air environment: Graphite is unsuitable. You must use a metallic element designed for oxidation resistance, such as a FeCrAl (Kanthal) or NiCr (Nichrome) alloy.
- If your primary focus is minimizing operational costs for a compatible process: Graphite offers significant savings in both material expense and energy efficiency, provided you can maintain the required non-oxidizing atmosphere.
Understanding these core principles empowers you to select a heating element based not on convention, but on the fundamental physics of your specific application.
Summary Table:
| Property | Graphite Heating Element | Traditional Metal Element |
|---|---|---|
| Max Operating Temp | Up to 2500°C | Typically < 1400°C |
| Strength at High Temp | Increases with temperature | Weakens and softens |
| Resistance to Thermal Shock | Excellent | Moderate to poor |
| Cost & Machinability | Lower cost, easy to machine | Higher cost, harder to machine |
| Suitable Atmosphere | Vacuum or inert gas only | Air or oxidizing environments |
Ready to enhance your high-temperature processes with the right heating solution? At KINTEK, we specialize in providing advanced lab equipment and consumables, including graphite heating elements tailored for vacuum and inert gas furnaces. Our expertise ensures you get efficient, durable, and cost-effective solutions for your laboratory needs. Contact us today to discuss how our products can optimize your specific application!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- Can graphite withstand heat? Unlocking its extreme 3,600°C potential in inert environments
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- Why is graphite used in furnaces? For Extreme Heat, Purity, and Efficiency



















