In short, inert gases are used anytime a non-reactive atmosphere is needed. The most common real-world examples include helium for lifting balloons and cooling MRI magnets, neon for creating vibrant advertising signs, and argon for protecting metal during welding and preserving the filament in traditional light bulbs.
The true value of an inert gas is not what it does, but what it doesn't do. Its chemical stability is its defining feature, used to create controlled environments free from the unwanted chemical reactions—like oxidation and combustion—that would otherwise occur.

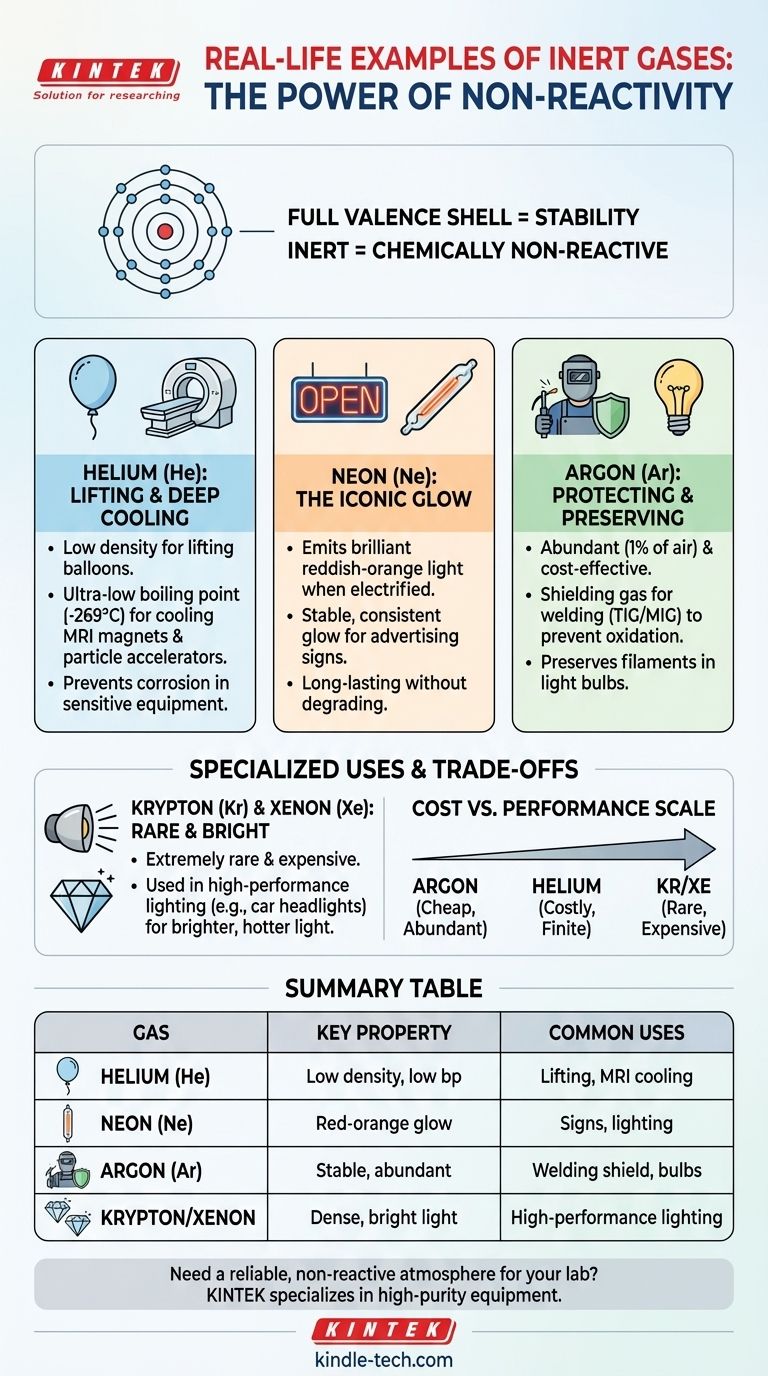
What Makes a Gas "Inert"?
The term "inert" describes a substance that is chemically non-reactive. In chemistry, this property is most famously embodied by the noble gases found in Group 18 of the periodic table.
The Secret is a Full Outer Shell
The stability of noble gases comes down to their atomic structure. They have a full outer shell of electrons, known as a valence shell.
This complete shell means they have little to no tendency to gain, lose, or share electrons with other elements. This fundamental lack of chemical ambition is what makes them so stable and predictable.
A Spectrum of "Inertness"
While we call them inert, their reactivity exists on a spectrum. Helium (He) and Neon (Ne) are extremely non-reactive.
However, heavier noble gases like Krypton (Kr), Xenon (Xe), and Radon (Rn) can be forced to form chemical compounds under very specific laboratory conditions. For all practical industrial and commercial purposes, however, they are treated as inert.
Key Examples in Technology and Industry
The non-reactivity of these gases makes them indispensable tools for solving specific engineering challenges.
Helium (He): Lifting and Deep Cooling
Helium is the second-lightest element. Because it is much less dense than air, its most famous use is for lifting weather balloons, scientific balloons, and party balloons.
Its most critical technical application, however, is as a liquid coolant. Liquid helium has an incredibly low boiling point (−452°F / −269°C), making it the ultimate refrigerant for cooling the superconducting magnets in MRI machines and particle accelerators like the Large Hadron Collider. Its inertness ensures it won't react with or corrode the sensitive equipment.
Neon (Ne): The Iconic Glow
When a high-voltage electrical current is passed through neon gas sealed in a glass tube, it emits a brilliant, stable, reddish-orange light. This is the principle behind iconic "neon" signs.
While other gases produce different colors (argon for blue, for example), neon's name has become synonymous with this type of lighting. Its inertness is key, as it allows the gas to glow consistently for thousands of hours without degrading.
Argon (Ar): Protecting and Preserving
Argon is the workhorse of inert gases because it makes up nearly 1% of Earth's atmosphere, making it abundant and cheap.
Its most common use is as a shielding gas in welding (like TIG and MIG welding). It's pumped over the weld area to displace oxygen and water vapor, which prevents oxidation and results in a much stronger, cleaner weld. The same principle is used in incandescent light bulbs, where argon fills the bulb to keep the hot tungsten filament from burning out.
Understanding the Trade-offs: Cost vs. Performance
The choice of which inert gas to use almost always comes down to balancing performance requirements with cost.
Abundance Dictates Price
Argon is cheap because it can be easily and economically separated from the air.
Helium is more expensive. While it's the second most abundant element in the universe, on Earth it is a finite resource, trapped underground and extracted with natural gas.
Krypton and Xenon are exceptionally rare in the atmosphere and very difficult to isolate, making them extremely expensive.
Matching the Gas to the Goal
You use argon for welding because it provides excellent protection at a low cost.
You might upgrade to a more expensive helium/argon mix only for specialized welding on highly thermally conductive metals like copper or aluminum.
Likewise, you use expensive xenon in high-performance car headlights because its density and properties allow the light to burn hotter and dramatically brighter, justifying the high cost for a premium application.
How to Recognize the Role of an Inert Gas
To understand why a specific inert gas is being used, consider the primary goal of the application.
- If the goal is to create a protective atmosphere: The gas, often argon, is being used to prevent an unwanted chemical reaction like oxidation (e.g., welding, food packaging, preserving historical documents).
- If the goal is specialized lighting: The choice (neon, argon, krypton, or xenon) is based on the desired color, brightness, and efficiency, with cost being a major factor in the decision.
- If the goal is extreme cooling or lifting: The unique physical properties of helium (low boiling point and low density) make it the only practical choice.
Ultimately, these invisible gases are critical enablers, allowing us to achieve feats of engineering and science that would be impossible in our reactive, oxygen-rich world.
Summary Table:
| Inert Gas | Key Property | Common Real-World Uses |
|---|---|---|
| Helium (He) | Low density, extremely low boiling point | Lifting balloons, cooling MRI magnets |
| Neon (Ne) | Emits red-orange light when electrified | Vibrant advertising signs, lighting |
| Argon (Ar) | Chemically stable, abundant in atmosphere | Welding shielding gas, preserving light bulb filaments |
| Krypton/Xenon | Dense, produces bright white light | High-performance lighting (e.g., car headlights) |
Need a reliable, non-reactive atmosphere for your laboratory processes? KINTEK specializes in providing high-purity lab equipment and consumables to meet your specific needs. Whether you require controlled environments for sensitive experiments or dependable supplies for your research, our expertise ensures precision and quality. Contact our team today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What is the importance of a laboratory electric constant temperature drying oven? Ensure Accurate Biomass Analysis
- What type of process is sintering? A Guide to Solid-State Fusion for Stronger Materials
- What are the technical requirements for vacuum chambers in desalination? Boost Efficiency with Graphene Technology
- What are the defects in sintered parts? Avoid Warping, Cracking, and Porosity Issues
- How does catalytic pyrolysis work? Unlock Higher Yields of Valuable Fuels and Chemicals
- What is the basic of brazing? A Guide to Strong, Low-Heat Metal Joining
- How many types of sputtering are there? A Guide to DC, RF, and Advanced Techniques
- What is the difference between thermal evaporation and molecular beam epitaxy? Choose the Right Thin-Film Deposition Method



















