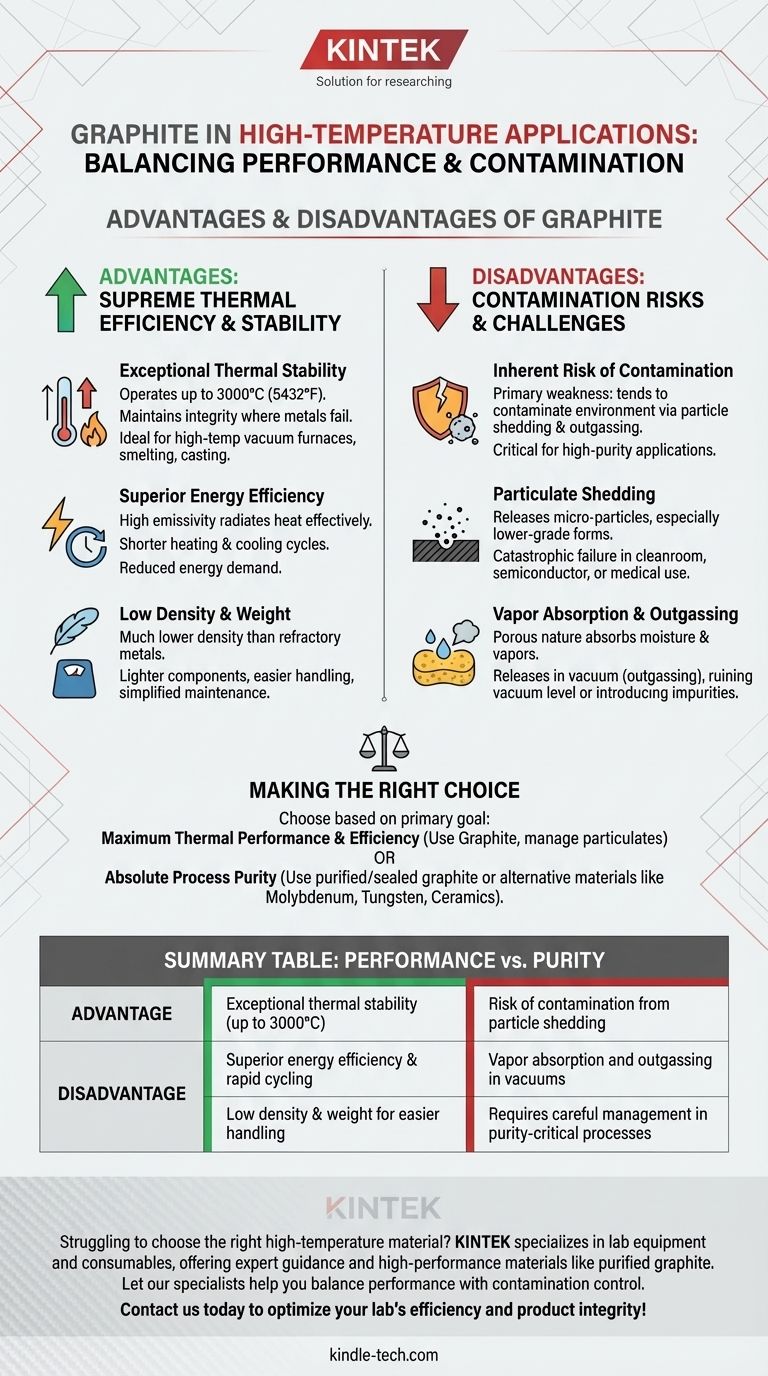
In high-temperature applications, graphite is a material of extremes. It offers unparalleled thermal performance, capable of operating at temperatures up to 3000°C where most metals would fail. Its key advantages are exceptional energy efficiency, low weight, and rapid heating and cooling cycles. However, its primary drawback is the significant risk of contamination from particle shedding and vapor absorption.
Graphite's core trade-off is clear: it provides supreme thermal efficiency and stability in extreme environments, but this performance comes at the cost of potential contamination, which must be carefully managed in sensitive processes.
The Core Advantages of Graphite in Demanding Environments
Graphite's unique atomic structure gives it a combination of properties that make it invaluable for specific, high-stress applications, particularly those involving intense heat.
Exceptional Thermal Stability
Graphite's most celebrated quality is its ability to perform at extreme temperatures. It maintains its structural integrity and properties in conditions reaching up to 3000°C (5432°F), far exceeding the operational limits of most metals and many ceramics.
This makes it an essential material for applications like high-temperature vacuum furnaces, smelting, and casting.
Superior Energy Efficiency
While graphite can absorb significant heat, its overall energy balance is remarkably efficient. It possesses high emissivity, meaning it radiates heat energy very effectively and uniformly.
This leads to shorter heating and cooling times, reducing overall process cycle durations and lowering the energy demand of equipment like induction furnaces.
Low Density and Weight
Compared to refractory metals that might be used in similar temperature ranges, graphite has a much lower density.
This makes components lighter, easier to handle, and reduces the structural support mass required in large-scale furnace construction. It also simplifies on-site repairs and replacement.
Understanding the Trade-offs: Graphite's Primary Disadvantages
The very properties that make graphite useful also introduce significant challenges. Its porous, layered nature is the source of its most critical disadvantages.
The Inherent Risk of Contamination
Graphite's primary weakness is its tendency to contaminate its operating environment. This happens in two primary ways: particle shedding and outgassing.
This single issue is often the deciding factor against its use in applications where purity is the top priority.
Particulate Shedding
Graphite is relatively soft and can release micro-particles from its surface, especially if it's a lower-grade or bonded form.
In cleanroom environments, semiconductor manufacturing, or medical applications, these free-floating carbon particles can cause catastrophic product failure or contamination.
Vapor Absorption and Outgassing
Graphite is porous and will absorb moisture, cleaning agents, and other vapors from the atmosphere. When heated, especially in a vacuum, it releases these trapped molecules in a process called outgassing.
This outgassing can ruin the vacuum level in a furnace or introduce unwanted chemical impurities into a sensitive process, compromising the integrity of the final product.
Making the Right Choice for Your Application
Selecting graphite requires balancing its unmatched thermal capabilities against the critical need to control contamination. Your primary goal will dictate whether it is the correct choice.
- If your primary focus is maximum thermal performance and energy efficiency: Graphite is an excellent, often necessary, choice for processes operating well above 2000°C where you can tolerate or manage minor carbon particulate.
- If your primary focus is absolute process purity and avoiding contamination: You must either invest in highly purified, sealed grades of graphite or consider alternative materials like molybdenum, tungsten, or advanced ceramics.
Ultimately, choosing graphite is an engineering decision that prioritizes its incredible heat tolerance while accepting the responsibility of mitigating its environmental impact on your process.
Summary Table:
| Advantage | Disadvantage |
|---|---|
| Exceptional thermal stability (up to 3000°C) | Risk of contamination from particle shedding |
| Superior energy efficiency & rapid cycling | Vapor absorption and outgassing in vacuums |
| Low density & weight for easier handling | Requires careful management in purity-critical processes |
Struggling to choose the right high-temperature material for your lab process? KINTEK specializes in lab equipment and consumables, providing expert guidance and high-performance materials like purified graphite to meet your specific thermal and purity requirements. Let our specialists help you balance performance with contamination control. Contact us today to optimize your lab's efficiency and product integrity!
Visual Guide
Related Products
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the industrial uses of graphite? Leverage Its Unique Properties for Demanding Applications
- Is graphite affected by heat? Discover Its Remarkable Strength and Stability at High Temperatures
- Why is graphite used for heat transfer? For Superior In-Plane Thermal Conductivity
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- What is special about graphite? Unlocking Its Unique Properties for Extreme Applications



















