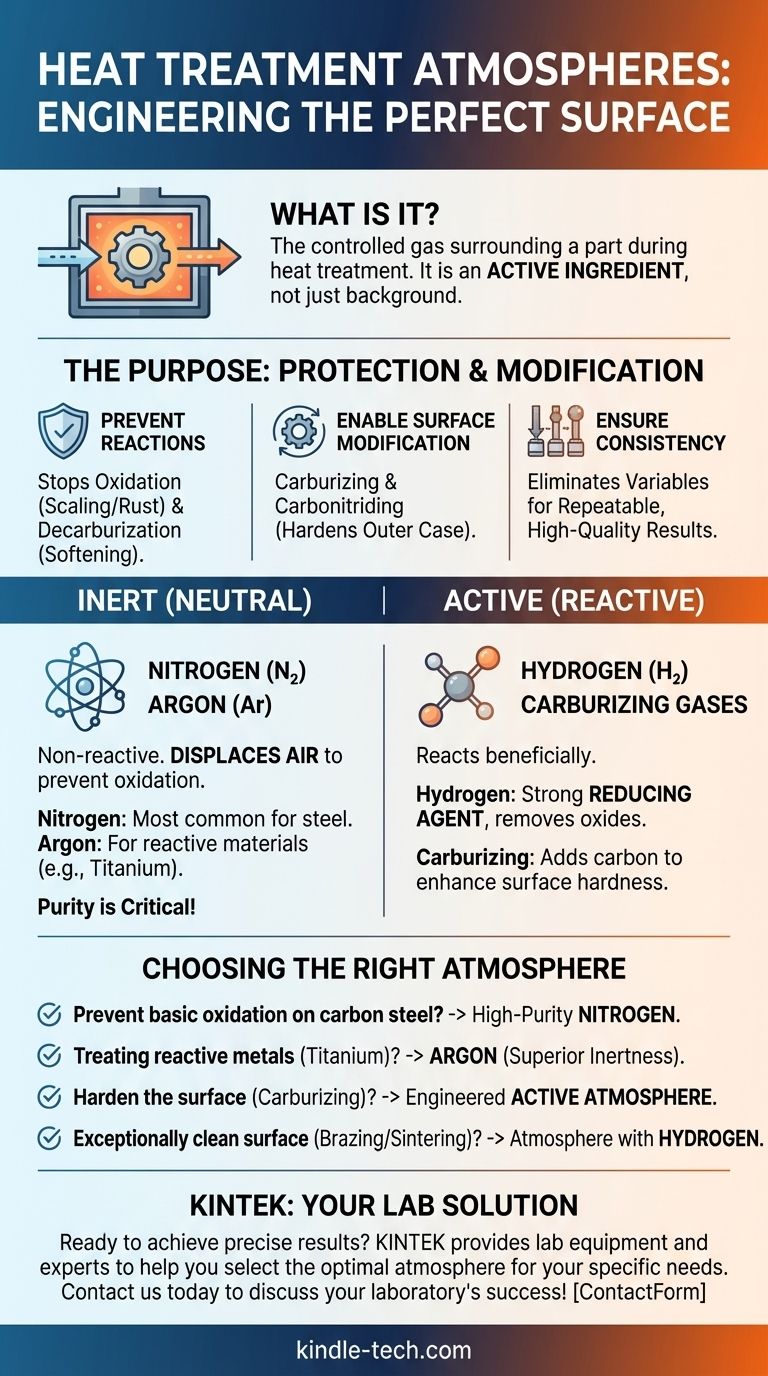
At its core, a heat treatment atmosphere is the controlled gas surrounding a part inside a furnace. These atmospheres are engineered to achieve specific outcomes, broadly classified into two types: inert atmospheres like nitrogen and argon that protect the part's surface, and active atmospheres containing gases like hydrogen or carbon dioxide that intentionally alter the surface.
The crucial insight is that the atmosphere is not merely a background element; it is an active ingredient in the heat treatment process. Your choice of atmosphere directly determines whether you are simply protecting a component or fundamentally engineering its surface for enhanced performance.

The Purpose of a Controlled Atmosphere
Using a controlled atmosphere instead of ambient air is a fundamental requirement for achieving high-quality, repeatable results in heat treatment. Air, composed primarily of nitrogen and oxygen, is highly reactive at elevated temperatures.
Preventing Unwanted Reactions
The primary goal of a protective atmosphere is to prevent unwanted chemical reactions on the material's surface.
The most common reaction is oxidation (scaling or rust), which occurs when a metal reacts with oxygen. Another is decarburization, the loss of carbon from the surface of steel, which softens the material and reduces its fatigue life.
Enabling Surface Modification
Active atmospheres are used to deliberately change the chemical composition of a part's surface.
Processes like carburizing and carbonitriding use atmospheres rich in carbon to diffuse it into the steel's surface, creating a hard, wear-resistant outer case while maintaining a tougher core.
Ensuring Consistency and Repeatability
By precisely controlling the gaseous environment, you eliminate the variables present in ambient air.
This control ensures that every part processed under the same conditions achieves the exact same metallurgical properties, which is critical for industries like aerospace and automotive.
A Breakdown of Common Atmospheres
Atmospheres are selected based on the material being treated and the desired outcome of the process. They can be sourced from on-site generators or pre-mixed synthetic gas supplies.
Inert (Neutral) Atmospheres
Inert atmospheres are chemically non-reactive with the workpiece material. Their sole purpose is to displace air and prevent oxidation and decarburization.
Nitrogen (N₂) is the most widely used inert gas for heat treating steel. It is effective, economical, and does not react with iron-based alloys.
Argon (Ar) is a more truly inert gas than nitrogen and is used for highly reactive materials like titanium, certain stainless steels, and non-ferrous metals. It is more expensive but provides superior protection.
The purity of an inert gas is critical. Low oxygen content and a very low dew point (a measure of moisture) are essential for preventing even microscopic levels of oxidation.
Active (Reactive) Atmospheres
Active atmospheres are designed to react with the workpiece in a controlled and beneficial way.
Hydrogen (H₂) is a strong reducing agent, meaning it actively removes oxides from a metal's surface. It is often mixed with nitrogen for processes like brazing and sintering to ensure an exceptionally clean surface.
Carburizing Atmospheres are generated to provide a specific "carbon potential." These mixtures can contain carbon monoxide (CO), carbon dioxide (CO₂), and hydrocarbons to control the diffusion of carbon into the steel.
Understanding the Trade-offs
The choice of atmosphere involves balancing cost, complexity, and the specific requirements of the material and process.
Cost vs. Performance
Nitrogen is the economic workhorse for most steel applications.
Argon provides the highest level of protection but comes at a significant cost premium, making it suitable only when absolutely necessary for reactive materials.
Generation vs. Supply
Gases can be produced on-site (e.g., nitrogen generators) or delivered in bulk liquid or high-pressure cylinder form. On-site generation can have a higher initial investment but lower long-term operating costs.
Safety and Complexity
Purely inert systems are relatively simple and safe.
Atmospheres containing reactive gases like hydrogen or carbon monoxide introduce flammability and toxicity risks, requiring more complex furnace controls and rigorous safety protocols.
Making the Right Choice for Your Goal
Selecting the correct atmosphere is a critical decision that directly impacts the quality, performance, and cost of the final component.
- If your primary focus is preventing basic oxidation on carbon steel: A high-purity nitrogen atmosphere is the most effective and economical solution.
- If you are treating reactive metals like titanium or specific stainless steels: Argon is the required choice for its superior inertness.
- If your goal is to harden the surface of steel (carburizing): You need a specifically engineered active atmosphere with a controlled carbon source.
- If you require an exceptionally clean, oxide-free surface for brazing or sintering: An atmosphere containing hydrogen is necessary to chemically reduce any existing surface oxides.
Ultimately, choosing the correct atmosphere transforms heat treatment from a simple heating process into a precise metallurgical engineering practice.
Summary Table:
| Atmosphere Type | Common Gases | Primary Purpose | Ideal For |
|---|---|---|---|
| Inert (Neutral) | Nitrogen (N₂), Argon (Ar) | Prevent oxidation & decarburization | Protecting carbon steel (N₂), reactive metals like titanium (Ar) |
| Active (Reactive) | Hydrogen (H₂), Carburizing gases | Modify surface chemistry | Carburizing steel, brazing, sintering for oxide-free surfaces |
Ready to achieve precise, high-quality results in your heat treatment process? The right atmosphere is critical for protecting your materials and engineering their surface properties. KINTEK specializes in lab equipment and consumables, providing solutions for all your laboratory heat treatment needs. Our experts can help you select the optimal atmosphere for your specific application, ensuring consistency, performance, and cost-effectiveness. Contact us today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the function of nitrogen gas in the annealing process? Ensure Oxidation-Free Heat Treatment
- What gases are used in annealing? Select the Right Atmosphere for Your Metal
- Why are atmosphere-controlled furnaces used for solid electrolyte impurities? Optimize Your Battery Research Now
- How does an inert gas flow system protect Magnetic Composite Carbon? Ensure Yield and Magnetic Utility
- Why is an atmosphere-controlled reduction experimental device required? Precision in Ore Pellet Swelling Analysis
- What conditions do industrial furnaces provide for biomass carbonization? Optimize Your Activated Carbon Production
- What role does the reducing protective gas play in Cu-SiOC hybrid ceramics? Ensure Conductivity via Active Reduction
- How does high-purity flowing Argon provide protection during Cr-Al-C annealing? Ensure Superior MAX Phase Integrity



















