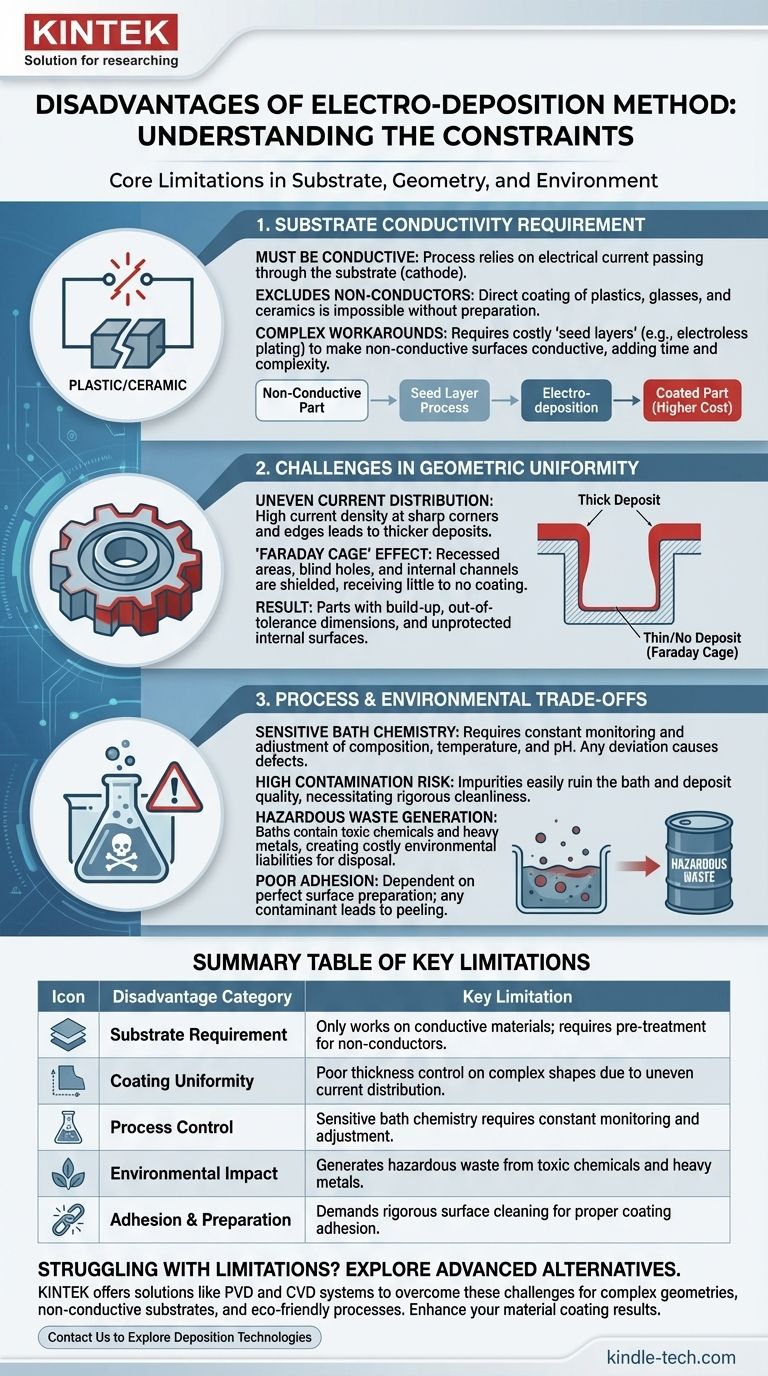
At its core, the primary disadvantages of the electrodeposition method are its requirement for a conductive substrate, its difficulty in producing uniform coatings on complex shapes, and the significant process control and environmental challenges associated with its chemical baths. While highly effective in specific contexts, these limitations make it unsuitable for many advanced materials and manufacturing applications.
Electrodeposition is a powerful and cost-effective technique, but its value is defined by its constraints. The method's reliance on electrical current and aqueous chemistry is both its strength and the source of its most significant drawbacks in geometry, material compatibility, and environmental safety.

The Fundamental Constraint: Substrate Conductivity
The entire process of electrodeposition, also known as electroplating, hinges on passing an electrical current through a conductive solution (the electrolyte) to a conductive part (the substrate). This creates a foundational limitation.
The Requirement for a Conductive Path
Electrodeposition works by reducing metal ions from the electrolyte solution onto the surface of the substrate, which acts as the cathode (negative electrode). If the substrate cannot conduct electricity, this circuit cannot be completed, and no deposition will occur.
This immediately excludes the direct coating of most plastics, ceramics, and glasses without additional, often complex, preparation steps.
Workarounds Add Complexity and Cost
To plate a non-conductive material, it must first be made conductive. This is typically done by applying a thin "seed layer" of conductive material through another process, like electroless plating or physical vapor deposition (PVD).
While effective, this adds significant time, cost, and complexity to the manufacturing process, potentially negating the primary cost advantages of electrodeposition.
The Challenge of Geometric Uniformity
The flow of electrical current is not uniform across a complex surface. This physical reality creates significant challenges in achieving a consistent coating thickness.
Uneven Current Distribution
Current density is naturally higher on sharp corners, edges, and protrusions. These high-current-density areas receive a much thicker deposit, while recessed areas, holes, and cavities receive a thinner one.
This can lead to parts that are out of tolerance, with built-up edges that may need post-processing and internal corners that lack sufficient protective coating.
The "Faraday Cage" Effect
Deep recesses, blind holes, or internal channels are effectively shielded from the electric field. This phenomenon, known as the Faraday cage effect, can prevent the deposition current from reaching these surfaces at all.
Consequently, it is extremely difficult to reliably coat the inside of complex components or intricate channels using standard electrodeposition methods.
Understanding the Process and Environmental Trade-offs
The chemical bath is the heart of the electrodeposition process, but it is also the source of major operational and environmental burdens.
Complex and Sensitive Bath Chemistry
The deposition quality is highly sensitive to the bath's composition, temperature, pH, and the concentration of metal ions and additives. These parameters must be constantly monitored and adjusted.
Any deviation can lead to defects in the coating, such as poor adhesion, brittleness, or incorrect thickness, making process control a constant and critical task.
High Risk of Contamination
The electrolyte bath is easily contaminated by impurities from the anodes, the substrate, or the surrounding environment. Even trace amounts of unwanted substances can ruin the entire bath and compromise the quality of the deposit.
This necessitates clean working conditions and rigorous quality control, adding to the operational overhead.
Poor Adhesion from Improper Preparation
The adhesion of the electrodeposited layer is entirely dependent on the cleanliness and preparation of the substrate surface. Any oils, oxides, or other contaminants will lead to a weak bond, causing the coating to peel, flake, or blister.
Surface preparation is a multi-step and resource-intensive process that is absolutely critical for success.
Generation of Hazardous Waste
Electroplating baths often contain heavy metals, cyanides, and other toxic chemicals. When the bath is spent or contaminated, it becomes hazardous waste that is costly and difficult to dispose of according to environmental regulations.
This presents a significant environmental liability and a major factor in the total cost of ownership for the process.
Making the Right Choice for Your Goal
Selecting a deposition method requires balancing cost, material properties, part geometry, and environmental impact.
- If your primary focus is cost-effective coating of simple, conductive metal parts: Electrodeposition is often the most economical and efficient choice.
- If your primary focus is coating complex 3D shapes or internal surfaces: You must account for thickness non-uniformity and consider alternatives like Chemical Vapor Deposition (CVD) or electroless plating.
- If your primary focus is depositing on non-conductive materials like plastics or ceramics: Electrodeposition is only feasible with an added conductive seed layer, making processes like Physical Vapor Deposition (PVD) a more direct alternative.
- If your primary focus is minimizing environmental impact and operational complexity: The costs and regulations associated with bath chemistry and waste disposal must be a central part of your evaluation.
Understanding these inherent limitations is the first step toward selecting a deposition strategy that aligns with your technical goals and operational realities.
Summary Table:
| Disadvantage Category | Key Limitation |
|---|---|
| Substrate Requirement | Only works on conductive materials; requires pre-treatment for non-conductors. |
| Coating Uniformity | Poor thickness control on complex shapes due to uneven current distribution. |
| Process Control | Sensitive bath chemistry requires constant monitoring and adjustment. |
| Environmental Impact | Generates hazardous waste from toxic chemicals and heavy metals. |
| Adhesion & Preparation | Demands rigorous surface cleaning for proper coating adhesion. |
Struggling with electrodeposition limitations for your lab's coating needs? KINTEK specializes in advanced lab equipment and consumables, offering solutions like PVD and CVD systems that overcome these challenges. Whether you're working with complex geometries, non-conductive substrates, or require eco-friendly processes, our expertise can enhance your material coating results. Contact us today to explore the right deposition technology for your laboratory!
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- What are the advantages of using HFCVD for BDD electrodes? Scaling Industrial Diamond Production Efficiently
- How does a Hot Filament Chemical Vapor Deposition (HFCVD) reactor function? Expert Guide to Diamond Film Fabrication
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating
- What is the role of the HF-CVD system in preparing BDD electrodes? Scalable Solutions for Boron-Doped Diamond Production



















