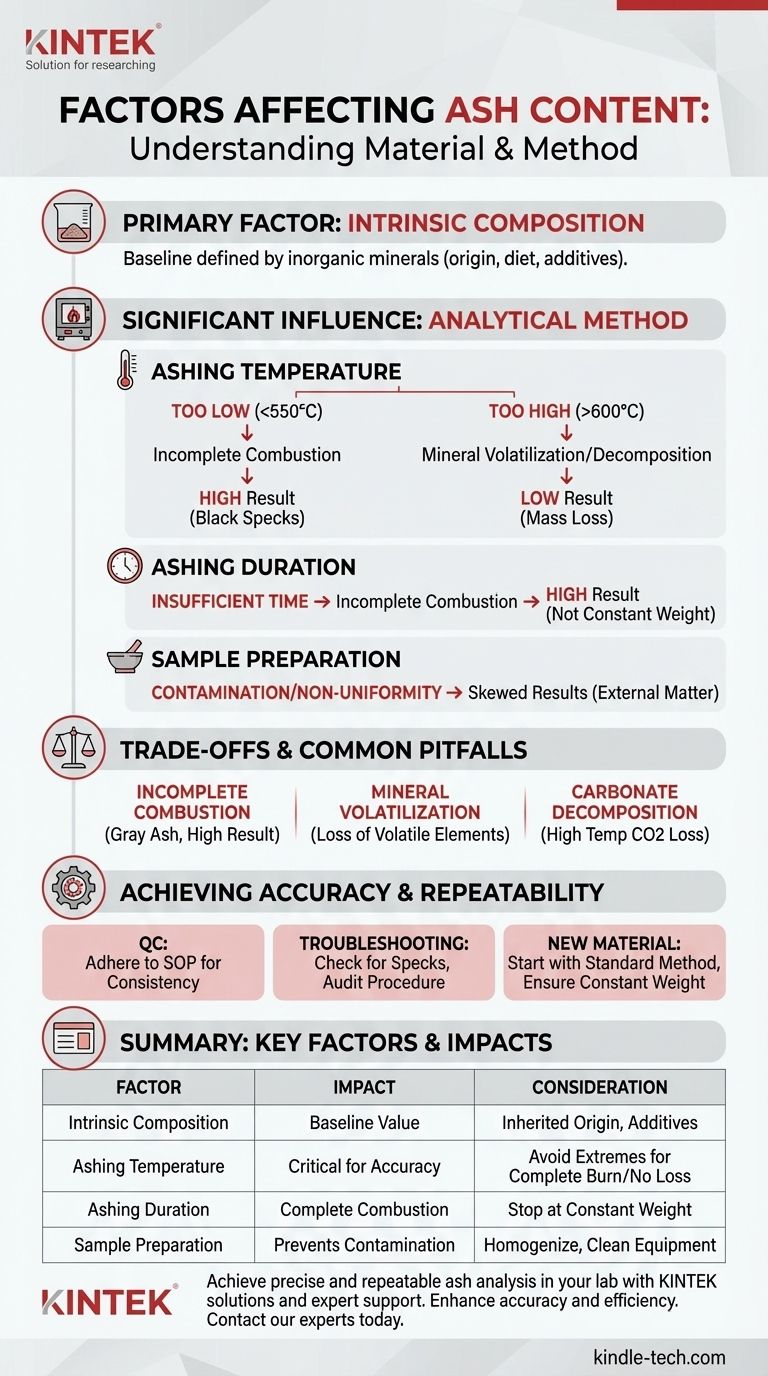
The primary factor determining ash content is the intrinsic amount of inorganic mineral matter within a sample. However, the final measured value is significantly influenced by the analytical method itself, particularly the temperature and duration of the combustion process, as well as how the sample is prepared.
While a material's ash content is fundamentally tied to its composition, the most common source of inconsistent results is not the material itself, but variations in the analytical procedure. Mastering the method is key to achieving accurate and repeatable measurements.

The Core Factor: Intrinsic Material Composition
The baseline ash content of any material is defined by the inorganic elements it contains. This composition is not random; it is a direct result of the material's origin and history.
Geological and Biological Origins
For natural products, the mineral content is inherited from its environment. A plant's ash content is a reflection of the soil minerals it absorbed, while the ash in a food product like milk is determined by the animal's diet and metabolism.
Processing and Additives
Manufacturing and processing can significantly alter ash content. Refining processes, like turning whole wheat into white flour, remove the mineral-rich bran and germ, lowering the ash value. Conversely, adding inorganic compounds like calcium carbonate (a whitening agent) or sodium chloride (salt) will increase the measured ash content.
How Analytical Methods Influence Results
The process of measuring ash, known as ashing or incineration, involves burning away all organic matter to isolate the inorganic residue. How this is performed has a profound impact on the result.
The Role of Ashing Temperature
Temperature is the most critical parameter. Most standard methods operate between 550°C and 600°C. If the temperature is too low, combustion may be incomplete, leaving carbon behind and falsely inflating the ash value.
If the temperature is too high, certain inorganic salts can decompose or volatilize (turn into a gas), leading to a loss of mass and a falsely low ash reading. Elements like chlorine, sodium, and potassium are particularly susceptible to this.
The Impact of Ashing Duration
The sample must be heated for a sufficient amount of time to ensure all organic material has been completely burned away. This is typically determined by heating until the sample reaches a "constant weight," meaning its weight no longer changes between successive measurements.
Cutting the time short is a common error that results in incomplete combustion and artificially high ash values.
The Importance of Sample Preparation
The sample must be uniform (homogenized) to ensure the small portion being analyzed is representative of the entire batch. Contamination from grinding equipment, unclean crucibles, or even the water used for cleaning can introduce external inorganic material, skewing results high.
Understanding the Trade-offs and Common Pitfalls
Achieving a "true" ash value involves balancing competing factors. It is less about finding one perfect number and more about achieving a consistent result through a standardized process.
Incomplete Combustion
The most frequent error in ash analysis is failing to burn off all the carbon. The resulting ash will appear gray or have black specks instead of being a uniform white or light gray powder. This always leads to a result that is higher than the actual value.
Volatilization of Minerals
This is the opposite problem. When trying to ensure complete combustion with higher heat, you risk losing volatile minerals. This trade-off is why standardized methods (like those from AOAC or ASTM) are so important; they define a precise temperature and time to create a repeatable result, even if it's not a theoretically "perfect" one.
Carbonate Decomposition
If a sample contains carbonates (like calcium carbonate), very high temperatures can cause them to decompose into oxides (e.g., calcium oxide) and release carbon dioxide gas. This loss of CO₂ mass will lead to an underestimation of the ash content. This is another reason why temperatures are typically capped around 600°C.
Achieving Accurate and Repeatable Ash Analysis
Your approach to ash analysis should be guided by your ultimate goal. Whether for quality control or research, understanding these factors is key to interpreting your data correctly.
- If your primary focus is routine quality control: Strict and unwavering adherence to a validated, standard operating procedure (SOP) is your most important task. Consistency is more critical than absolute accuracy.
- If your primary focus is seeing inconsistent results: First, visually inspect your ashed samples for black specks (incomplete combustion). If none are present, audit your procedure for variations in temperature, time, and crucible cleaning.
- If your primary focus is characterizing a new material: Begin with a standard method (e.g., 550°C for several hours) and check for constant weight. This provides a reliable baseline for comparison against other materials.
By controlling these factors, you transform ash analysis from a simple measurement into a powerful tool for understanding your material's quality and composition.
Summary Table:
| Factor | Impact on Ash Content | Key Consideration |
|---|---|---|
| Intrinsic Composition | Defines the baseline value | Inherited from origin (soil, diet, additives) |
| Ashing Temperature | Critical for accuracy | Too low: incomplete combustion (high result). Too high: mineral loss (low result) |
| Ashing Duration | Ensures complete combustion | Stop at constant weight; insufficient time inflates result |
| Sample Preparation | Prevents contamination | Homogenize sample; use clean equipment to avoid skewing results |
Achieve precise and repeatable ash analysis in your lab. Inconsistent results can stem from subtle variations in your procedure. KINTEK specializes in providing reliable lab equipment and expert support to help you master your ashing method. Whether you're in food science, pharmaceuticals, or materials testing, our solutions are designed for your specific needs. Contact our experts today to discuss how we can enhance your analytical accuracy and efficiency.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of muffle furnace in food industry? Essential for Accurate Food Ash Analysis
- Can calcination be done in a muffle furnace? Yes, for precise air-atmosphere heating.
- How do you control a muffle furnace? Master Precise Temperature Control for Your Lab
- What hazard is involved when using a furnace? Protect Your Home from the Silent Killer
- What are the conditions for a muffle furnace? Ensure Safety, Performance, and Longevity



















