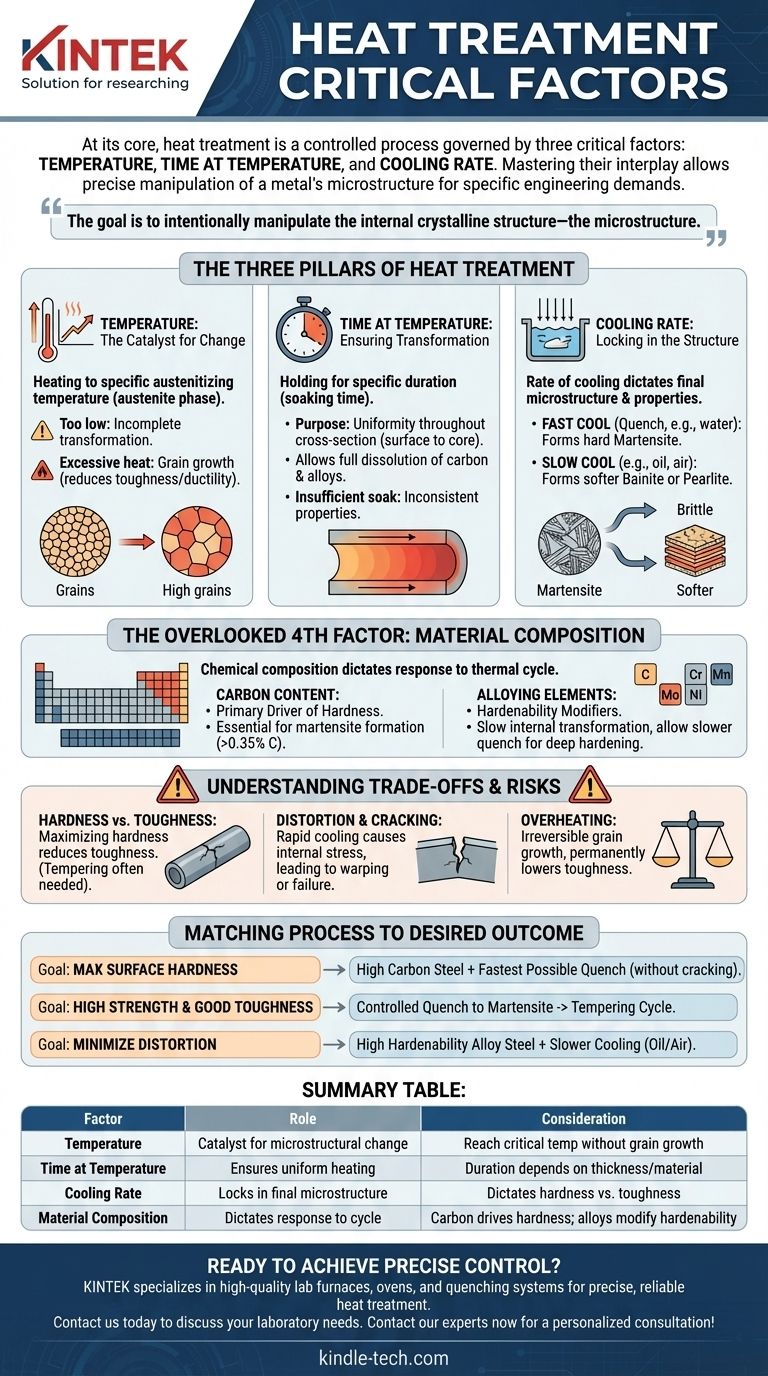
At its core, heat treatment is a controlled process governed by three critical factors: temperature, time at temperature, and the cooling rate. Mastering the interplay between these variables is what allows you to precisely manipulate the mechanical properties of a metal to meet specific engineering demands.
The goal of heat treatment isn't simply to heat and cool a metal; it's to intentionally manipulate its internal crystalline structure—the microstructure. Each factor serves a distinct purpose in this transformation, and a failure in one can compromise the entire process.

The Three Pillars of Heat Treatment
The success of any heat treatment cycle depends on the precise control of three fundamental variables. They are sequential and interdependent, with each stage setting the foundation for the next.
Temperature: The Catalyst for Change
The first step is heating the material to a specific austenitizing temperature. This is the temperature at which the steel's crystal structure transforms into a phase known as austenite, which is capable of dissolving carbon.
Reaching this critical temperature is non-negotiable. If the temperature is too low, the transformation will be incomplete, and the desired properties cannot be achieved upon cooling.
However, significantly overshooting this temperature is detrimental. Excessive heat causes the individual crystalline grains to grow, a condition known as grain growth, which can permanently reduce the material's toughness and ductility.
Time at Temperature: Ensuring Transformation
Once the material reaches the target temperature, it must be held there for a specific duration. This is known as the soaking time.
The primary purpose of soaking is to ensure uniformity. It allows the temperature to become consistent throughout the entire cross-section of the part, from the surface to the core.
Soaking also provides the necessary time for the carbon and alloying elements to fully dissolve into the austenite structure. Insufficient soak time, especially in thicker components, results in an incomplete transformation and inconsistent properties.
Cooling Rate: Locking in the Structure
The rate at which the material is cooled from the austenitizing temperature is arguably the most critical factor. This rate dictates the final microstructure and, therefore, the material's final mechanical properties.
A very fast cool, or quench (e.g., in water or brine), traps the carbon atoms, forming a hard and brittle structure called martensite. This is essential for achieving maximum hardness.
Slower cooling rates (e.g., in oil or air) allow for the formation of softer, more ductile structures like bainite or pearlite. The choice of quenching medium is the primary tool for controlling this rate.
The Overlooked Fourth Factor: Material Composition
The three pillars of heat treatment do not operate in a vacuum. The chemical composition of the metal itself dictates how it will respond to the thermal cycle.
Carbon Content: The Primary Driver of Hardness
Carbon is the single most important element for the hardenability of steel. Without a sufficient amount of carbon (typically above 0.35%), the formation of hard martensite is impossible, no matter how rapid the quench.
Alloying Elements: The Hardenability Modifiers
Alloys like chromium, manganese, molybdenum, and nickel play a crucial role. They slow down the internal transformation, making it possible to achieve a hardened structure with a slower, less severe quench.
This property, known as hardenability, is vital for heat-treating thick sections. It allows the core of a large component to harden without requiring a quench so drastic that it would crack or distort the surface.
Understanding the Trade-offs and Risks
Achieving the desired outcome requires balancing competing properties and mitigating inherent risks.
The Hardness vs. Toughness Dilemma
Maximizing hardness almost always comes at the expense of toughness. The martensitic structure that provides extreme hardness is also very brittle. This is why a secondary heat treatment process called tempering is almost always performed after quenching to restore some toughness.
The Risk of Distortion and Cracking
Rapid cooling is a violent process that creates immense internal stress as different parts of the component cool and shrink at different rates. This stress can cause the part to warp (distortion) or, in severe cases, to fail catastrophically (quench cracking).
The Danger of Overheating
As mentioned, heating a part too far above its critical temperature causes irreversible grain growth. Large-grained steel has significantly lower toughness and impact resistance. This is a common and costly error that cannot be corrected by subsequent heat treatment.
Matching the Process to the Desired Outcome
Your heat treatment strategy must be dictated by your end goal. The "correct" parameters are entirely dependent on the desired performance of the component.
- If your primary focus is maximum surface hardness: You need a steel with sufficient carbon content and the fastest possible quench rate that the part's geometry can survive without cracking.
- If your primary focus is high strength combined with good toughness: You will use a controlled quench to achieve a fully hardened (martensitic) structure, followed by a specific tempering cycle to reduce brittleness.
- If your primary focus is minimizing distortion in complex parts: You should use an alloyed steel with high hardenability, allowing you to use a slower cooling medium (like oil or even air) to achieve the necessary hardness.
Ultimately, mastering heat treatment is about understanding that you are precisely controlling the formation of a material's internal structure.
Summary Table:
| Factor | Role in Heat Treatment | Key Consideration |
|---|---|---|
| Temperature | Catalyst for microstructural change (austenitizing) | Must reach critical temperature without causing grain growth |
| Time at Temperature | Ensures uniform heating and complete transformation (soaking) | Duration depends on part thickness and material |
| Cooling Rate | Locks in the final microstructure and properties (quenching) | Dictates hardness (fast quench) vs. toughness (slow quench) |
| Material Composition | Dictates response to the thermal cycle (the 4th factor) | Carbon content drives hardness; alloys modify hardenability |
Ready to Achieve Precise Control Over Your Heat Treatment Processes?
Mastering the critical factors of temperature, time, and cooling rate is essential for developing materials with the exact properties you need. KINTEK specializes in providing high-quality lab furnaces, ovens, and quenching systems that deliver the precise, reliable control required for successful heat treatment cycles.
Whether you are focused on maximizing hardness, improving toughness, or minimizing distortion in complex parts, the right equipment is key. Contact us today to discuss your specific laboratory needs and discover how our solutions can enhance your research and production outcomes.
Contact our experts now for a personalized consultation!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the temperature of HVAC brazing? Master the Perfect Joint for Leak-Proof Systems
- Why is argon gas used during the sputtering of a metal? Achieve High-Quality, Contamination-Free Thin Films
- What are the guidelines to follow while heating substances in the laboratory? Ensure Safe and Controlled Heating Processes
- What are the disadvantages of RF magnetron sputtering? Key Limitations for Thin Film Deposition
- Why are vacuum drying and argon purging equipment necessary for SILP catalyst impregnation? Optimize Performance Now
- What are the challenges of biomass conversion? Overcoming Economic and Technical Hurdles
- How do you calculate the capacity of a filter press? Use Pilot Testing for Accurate Sizing
- What is the process of DC sputtering? A Step-by-Step Guide to Thin Film Deposition



















