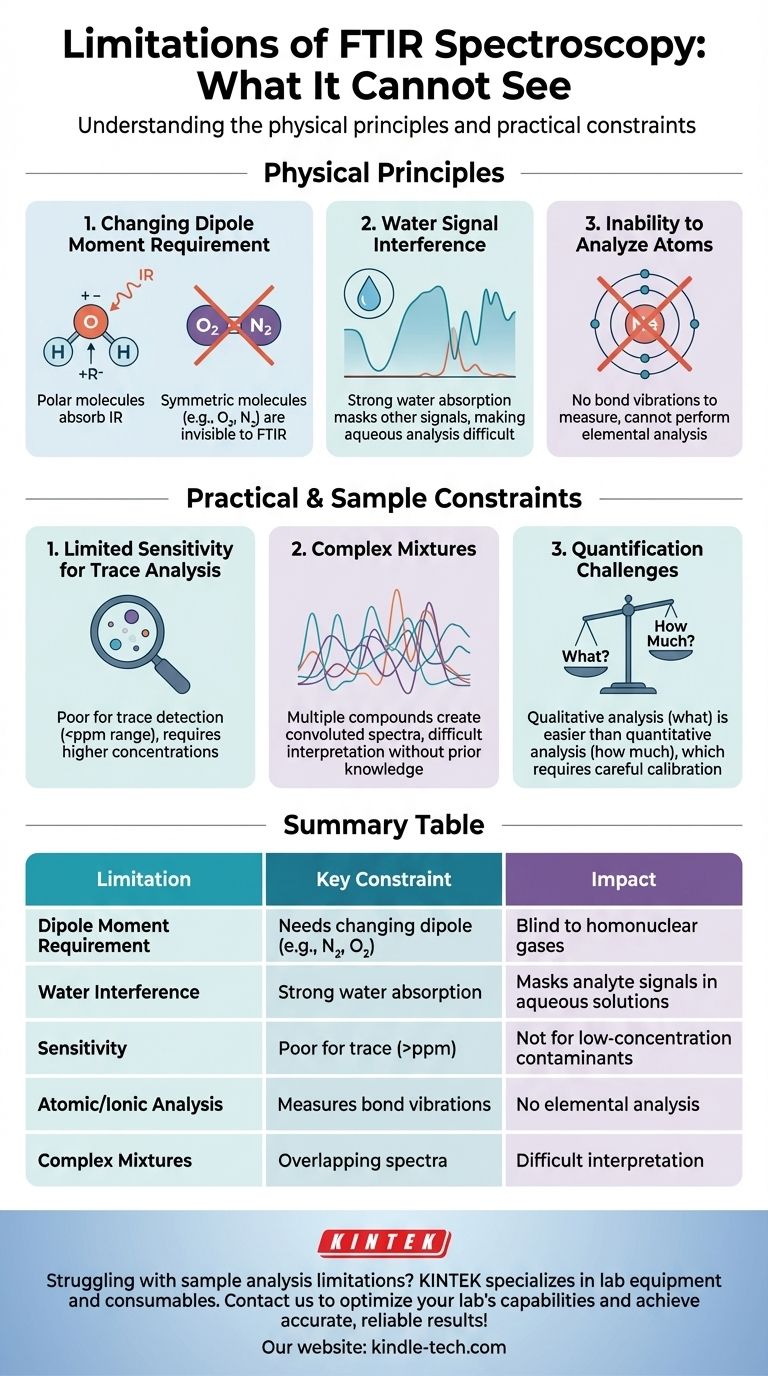
While incredibly powerful for molecular identification, the primary limitations of Fourier Transform Infrared (FTIR) spectroscopy are its poor sensitivity for trace analysis, its inability to analyze samples with high water content due to signal interference, and its fundamental blindness to molecules that do not exhibit a change in dipole moment during vibration, such as homonuclear diatomic molecules (e.g., O₂, N₂). It is also unable to provide information about individual atoms or atomic ions.
The core challenge of FTIR is not what it does, but what it cannot see. Its power lies in identifying the functional groups that make up molecules, but it struggles when a sample is too dilute, dissolved in water, or composed of molecules that are transparent to infrared radiation.
The Physical Principles Behind the Limitations
To understand the constraints of FTIR, we must first understand its mechanism. The technique works by measuring the absorption of infrared light by a molecule, which only occurs if the molecule's vibration or rotation causes a change in its net dipole moment.
The Requirement for a Changing Dipole Moment
A molecule must have a changing dipole moment to absorb infrared radiation. This is a fundamental selection rule of the technique.
Homonuclear diatomic molecules like nitrogen (N₂) and oxygen (O₂), which constitute most of the air, have a symmetric charge distribution. Their vibrations do not create a charge imbalance, so they have no changing dipole moment and are therefore IR-inactive, or invisible to FTIR.
The Overwhelming Signal of Water
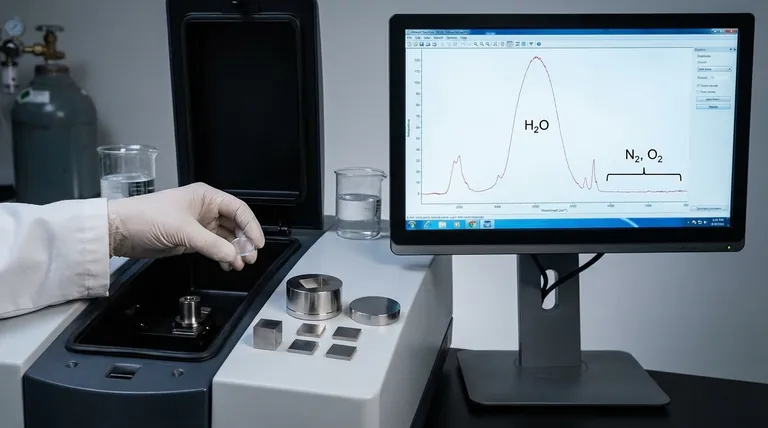
Water (H₂O) is a polar molecule that absorbs infrared radiation very strongly across a broad range of the spectrum.
If an analyte is dissolved in water, the intense absorption bands from the water can completely overwhelm or mask the much weaker signals from the substance you are trying to analyze. This makes analyzing aqueous solutions with standard transmission FTIR nearly impossible without specialized methods.
Inability to Analyze Atoms
FTIR spectroscopy measures the vibrational energy between bonds connecting atoms.
Single atoms (like noble gases or metal ions) do not have chemical bonds that can vibrate in this manner. Consequently, FTIR cannot be used for elemental analysis.
Practical and Sample-Related Constraints
Beyond the fundamental physics, several practical challenges can limit the effectiveness of FTIR for certain applications.
Limited Sensitivity for Trace Analysis
FTIR is generally considered a bulk analysis technique, not a trace analysis technique.
While specialized setups can push detection limits, it typically requires concentrations well above the parts-per-million (ppm) range. Techniques like gas or liquid chromatography paired with mass spectrometry (GC-MS, LC-MS) are far more suitable for detecting trace contaminants.
Challenges with Complex Mixtures
When analyzing a sample containing many different compounds, their individual infrared spectra will overlap.
This creates a complex, convoluted spectrum that can be extremely difficult to interpret and to assign specific peaks to specific components without advanced statistical software or prior knowledge of the sample's composition.
Quantification Can Be Difficult
While FTIR can be used for quantitative analysis (determining "how much"), it is often less straightforward than qualitative analysis (determining "what is present").
This requires careful creation of a calibration curve based on standards, and it relies on the Beer-Lambert law, which can deviate at high concentrations. This process can be time-consuming and prone to error if the sample matrix is complex.
Understanding the Trade-offs
Choosing an analytical technique always involves balancing its strengths and weaknesses. FTIR is no exception.
Speed vs. Specificity
FTIR provides a molecular "fingerprint" almost instantly, making it excellent for rapid quality control or screening. However, that fingerprint represents the collection of functional groups, not necessarily the complete, unambiguous structure of a single molecule, which is better provided by techniques like Nuclear Magnetic Resonance (NMR).
Qualitative Strength vs. Quantitative Challenge
FTIR is exceptionally powerful for quickly identifying the types of chemical bonds and functional groups present in a sample. It answers the "what is it?" question very well. Answering the "how much is there?" question requires significantly more effort and calibration.
Non-Destructive vs. Limited Scope
A major advantage of FTIR is that it is a non-destructive technique, meaning the sample can be recovered and used for other analyses. The trade-off is that the information is limited to vibrational properties; you get no data on molecular weight, elemental composition, or electronic structure.
Is FTIR the Right Tool for Your Analysis?
Use these guidelines to determine if FTIR is the appropriate choice for your specific goal.
- If your primary focus is rapid identification of functional groups in pure or simple solid/liquid samples: FTIR is an outstanding first-line analytical tool.
- If your primary focus is analyzing samples in aqueous solutions: You must use a specialized technique like Attenuated Total Reflectance (ATR-FTIR) or consider an alternative method like Raman spectroscopy, which is insensitive to water.
- If your primary focus is trace-level detection of contaminants: You should evaluate more sensitive techniques, such as chromatography coupled with mass spectrometry.
- If your primary focus is determining the complete, unambiguous structure of an unknown molecule: FTIR is only one piece of the puzzle and must be combined with other methods like NMR and Mass Spectrometry.
By understanding these limitations, you can leverage the distinct strengths of FTIR effectively and make informed decisions about when to apply it or when to turn to a more suitable technique.
Summary Table:
| Limitation | Key Constraint | Impact on Analysis |
|---|---|---|
| Dipole Moment Requirement | Cannot analyze molecules without a changing dipole (e.g., N₂, O₂) | Blind to homonuclear diatomic gases |
| Water Interference | Strong absorption masks analyte signals in aqueous solutions | Difficult to analyze samples with high water content |
| Sensitivity | Poor for trace analysis (typically > ppm range) | Not suitable for detecting low-concentration contaminants |
| Atomic/Ionic Analysis | Measures bond vibrations, not individual atoms | Cannot perform elemental analysis |
| Complex Mixtures | Overlapping spectra from multiple compounds | Difficult interpretation without prior knowledge or advanced software |
Struggling with sample analysis limitations? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the right analytical tools—from FTIR accessories to complementary techniques like Raman spectroscopy—to overcome your specific challenges. Contact us today to optimize your lab's capabilities and achieve accurate, reliable results!
Visual Guide
Related Products
- Square Lab Press Mold for Laboratory Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
People Also Ask
- What is the temperature of a plasma arc furnace? Achieve Extreme Heat for Advanced Materials & Waste Destruction
- Why KBr is used to prepare samples for FTIR analysis? Unlock Clear, High-Quality Spectra
- Is carbon fiber filament electrically conductive? A Guide to ESD-Safe 3D Printing
- What is the role of a high-pressure homogenizer in PHA extraction? Optimize Your Bio-Material Recovery Process
- What can sintering affect? Transform Powder into High-Performance Solid Parts
- Why must Ni–20Cr–5Al alloy samples be dried in a laboratory oven? Ensure 0.0001g Precision in Corrosion Experiments
- Is heat treatment necessary? A Guide to Making the Right Engineering Choice
- What is the standard for melting point calibration? Ensure Accuracy with Certified Reference Materials


















