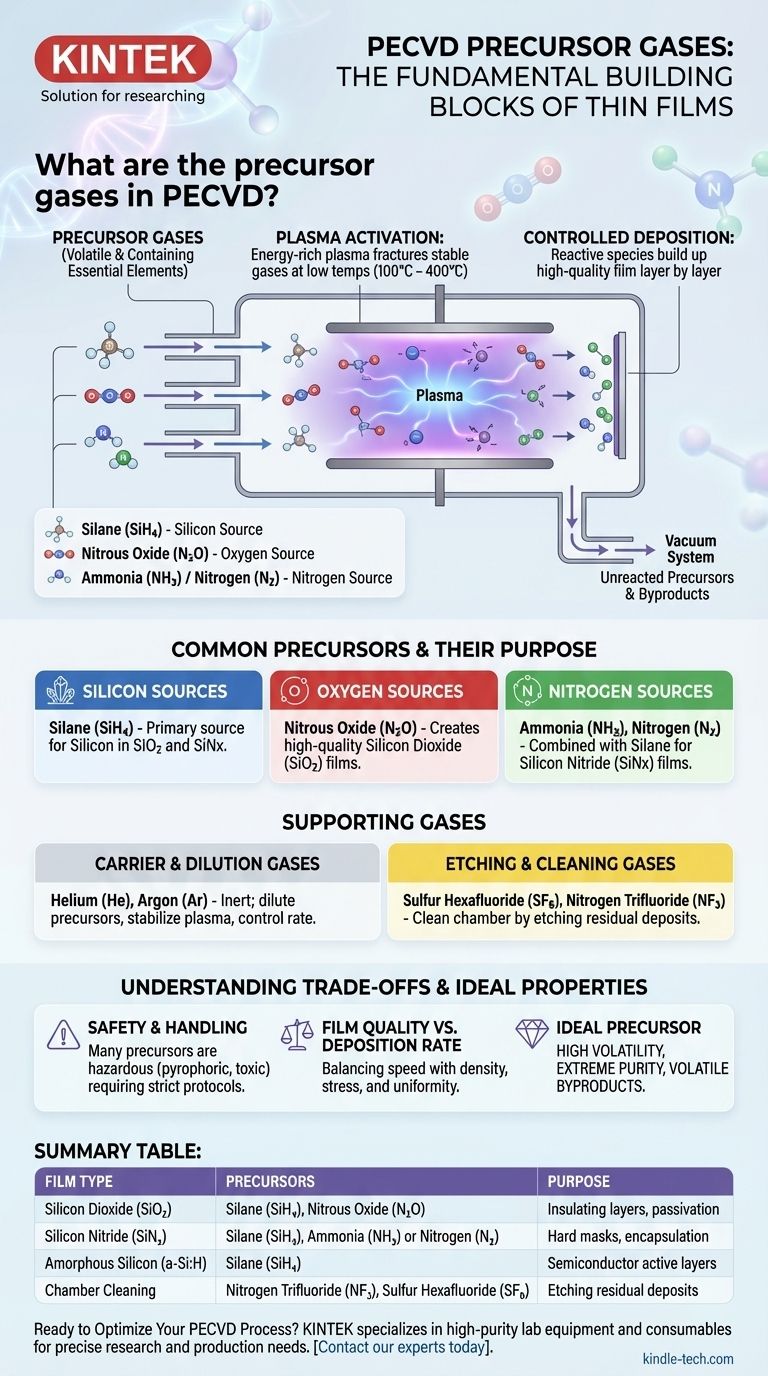
In Plasma-Enhanced Chemical Vapor Deposition (PECVD), precursor gases are the fundamental building blocks used to create thin films. Common examples include Silane (SiH4) for silicon, Nitrous Oxide (N2O) for oxygen, and Ammonia (NH3) or Nitrogen (N2) for nitrogen. These gases are chosen because they contain the necessary elements for the final film and are volatile enough to be introduced into a vacuum chamber.
The core principle is not just which gases are used, but how they are used. PECVD employs an energy-rich plasma to break down these stable precursor gases at low temperatures, enabling the controlled deposition of high-quality materials like silicon dioxide and silicon nitride onto a substrate.

The Role of Precursors in the PECVD Process
What Makes a Gas a "Precursor"?
A precursor is a volatile chemical compound that serves as the source material for the film you intend to deposit. In PECVD, these materials are introduced into a reaction chamber in gaseous form.
The process relies on plasma—a partially ionized gas created by applying a strong radio frequency (RF) electric field.
How Plasma Activates Precursors
Unlike traditional Chemical Vapor Deposition (CVD) which requires very high temperatures (over 600°C) to break chemical bonds, PECVD uses the energy of the plasma.
The free electrons in the plasma collide with the precursor gas molecules, fracturing them into highly reactive ions and radicals. This activation step allows the deposition reactions to occur at much lower temperatures, typically between 100°C and 400°C.
From Gas to Solid Film
Once broken down, these reactive species travel to the surface of the target substrate. There, they react and bond, gradually building up the desired solid thin film layer by layer.
Any unreacted precursors or gaseous byproducts are removed from the chamber by a vacuum system.
Common Precursors and Their Purpose
The specific precursor gases chosen directly determine the chemical composition of the final film. They are often used in combination.
Silicon Sources
Silane (SiH4) is the most common precursor for depositing any film containing silicon. It serves as the primary source for the "Si" in materials like silicon dioxide and silicon nitride.
Oxygen Sources
To deposit oxides, an oxygen-containing gas is required. Nitrous Oxide (N2O) is a widely used and effective oxygen source for creating high-quality silicon dioxide (SiO2) films.
Nitrogen Sources
For nitride films, a nitrogen source is combined with silane. Ammonia (NH3) and Nitrogen (N2) gas are the most common choices for depositing silicon nitride (SiNx).
Carrier and Dilution Gases
Inert gases like Helium (He) and Argon (Ar) do not participate in the chemical reaction. They are used to dilute the reactive precursors, stabilize the plasma, and control the deposition rate and film properties.
Etching and Cleaning Gases
Certain gases are used not for deposition, but for cleaning the inside of the reaction chamber between runs. Fluorine-based compounds like Sulfur Hexafluoride (SF6) and Nitrogen Trifluoride (NF3) are used to etch away residual film deposits.
Understanding the Trade-offs
Choosing the right precursors and process conditions involves balancing several critical factors.
Safety and Handling
Many precursor gases are hazardous. Silane, for example, is pyrophoric, meaning it can spontaneously ignite on contact with air. Others are toxic or corrosive, requiring stringent safety protocols and specialized handling equipment.
Film Quality vs. Deposition Rate
There is often a trade-off between the speed of deposition and the final quality of the film. High gas flows and plasma power can increase the deposition rate but may lead to films with lower density, higher stress, or poor uniformity.
The Properties of an Ideal Precursor
An ideal precursor is highly volatile, ensuring it can be easily transported into the chamber. It must also be extremely pure, as any contaminants in the gas can become incorporated into the film, degrading its performance. Finally, its reaction byproducts must also be volatile so they can be easily pumped away without contaminating the chamber.
Making the Right Choice for Your Goal
The combination of precursors is tailored to the specific film being created.
- If your primary focus is depositing Silicon Dioxide (SiO2): Your precursors will be a silicon source like Silane (SiH4) and an oxygen source like Nitrous Oxide (N2O).
- If your primary focus is depositing Silicon Nitride (SiNx): You will combine Silane (SiH4) with a nitrogen source, most commonly Ammonia (NH3) or N2 gas.
- If your primary focus is depositing Amorphous Silicon (a-Si:H): You will use Silane (SiH4) as the primary precursor, often diluted in a carrier gas like Argon or Helium.
- If your primary focus is chamber cleaning: You will use a fluorine-based gas like NF3 or SF6 to etch away residual material after deposition runs.
Ultimately, the selection of precursor gases is the foundational decision that dictates the chemistry of your thin film deposition process.
Summary Table:
| Film Type | Common Precursor Gases | Purpose |
|---|---|---|
| Silicon Dioxide (SiO₂) | Silane (SiH₄), Nitrous Oxide (N₂O) | Insulating layers, passivation |
| Silicon Nitride (SiNₓ) | Silane (SiH₄), Ammonia (NH₃) or Nitrogen (N₂) | Hard masks, encapsulation |
| Amorphous Silicon (a-Si:H) | Silane (SiH₄) | Semiconductor active layers |
| Chamber Cleaning | Nitrogen Trifluoride (NF₃), Sulfur Hexafluoride (SF₆) | Etching residual deposits |
Ready to Optimize Your PECVD Process?
The right precursor gases are fundamental to achieving high-quality, uniform thin films. KINTEK specializes in providing high-purity lab equipment and consumables for PECVD and other deposition techniques, serving the precise needs of research and production laboratories. Our expertise ensures you have the reliable materials and support necessary for successful deposition.
Contact our experts today to discuss your specific application and discover how we can help you achieve superior film quality and process efficiency.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vertical Laboratory Tube Furnace
People Also Ask
- What temperature is PECVD nitride? Optimize Your Silicon Nitride Film Properties
- What are the different types of Plasma Enhanced Chemical Vapour Deposition (PECVD)? Compare RF, VHF, and Microwave
- What is Microwave Electron Cyclotron Resonance Plasma Enhanced Chemical Vapour Deposition (MWECR-PECVD)? | KINTEK
- How does plasma vapor deposition work? A Low-Temperature Coating Solution for Sensitive Materials
- What process factors influence PECVD film quality? Mastering Energy, Pressure, and Temperature for Superior Growth
- How much does DLC coating cost? A Detailed Breakdown of Pricing Factors
- What are the advantages of PECVD over CVD? Achieve High-Quality Thin Films at Lower Temperatures
- How does plasma enhanced CVD work? Achieve Low-Temperature, High-Quality Thin Film Deposition



















